颈椎后纵韧带骨化症(OPLL)是一种常见的颈椎退行性疾病,也是导致颈脊髓压迫的常见原因,其在亚洲人群中的发生率为0.4%~3.0%[1-2]。随着韧带骨化灶的不断生长,患者的神经症状也会进行性加重,常需进行手术治疗。目前,治疗颈椎OPLL的手术方式主要为颈椎前路直接切除骨化物、颈椎后路椎板切除术和颈椎后路椎管扩大椎板成形术等。传统前路或后路手术各有其优缺点,前路具有较好的手术效果,后路具有较少的手术并发症。颈椎前路椎体骨化物复合体前移融合术(ACAF)[3-4]是一种全新的治疗颈椎OPLL的手术方式,该术式通过将椎体骨化物复合体整体前移达到扩大椎管、减压脊髓的目的。2017年5月—2017年8月,本研究组行ACAF治疗颈椎OPLL患者13例,现对ACAF术后颈椎椎管矢状径、椎管横截面积及椎管狭窄率的变化进行分析。
1 资料与方法 1.1 一般资料本组13例患者,男8例,女5例;年龄50~78岁,平均63.9岁。术中共前移椎体36个,其中前移C3~6椎体3例,前移C4~6椎体4例,前移C5~7椎体2例,前移C3~5椎体1例,前移C3椎体2例,前移C5椎体1例。其中有3例患者曾行颈椎后路全椎板切除术或颈椎后路半椎板切除减压内固定术,1例患者伴有颈椎过伸伤。所有患者的症状、体征与影像学检查均符合颈椎OPLL的诊断。
1.2 手术方法采用Smith-Peterson入路显露椎前间隙,做颈前纵或横切口。逐层显露至椎前间隙后透视确定手术节段无误。用三关节咬骨钳咬除目标间隙及椎体前缘骨赘,依次使用尖刀、刮匙和髓核钳彻底去除椎间盘,用枪钳咬除椎间隙后缘增生骨赘,显露后纵韧带。使用神经剥离钩寻找并突破后纵韧带的薄弱点,然后挑起后纵韧带并用尖刀切开。使用刮匙及枪钳咬除椎间隙后纵韧带,显露硬膜。仅切除头尾两端椎间隙的后纵韧带,骨化物所在的各节段椎间隙后纵韧带无需切除。使用三关节咬骨钳根据各节段骨化物厚度去除椎体前部部分骨质。对前部骨质去除不足的椎体使用磨钻或超声骨刀进行修整。根据试模测量各椎间隙大小,于各间隙安装填塞有自体骨的椎间融合器。将预弯的长度合适的钛板放置于椎体前缘,用钻头及丝攻预处理钉道后安装椎体钉。根据术前测量的骨化物宽度,向外1.0 mm作为开槽边界,一般开槽宽度为18.0~20.0 mm,可使用高速磨钻、超声骨刀或咬骨钳进行开槽,槽宽1.5~2.0 mm,至椎体后壁皮质后使用1 mm枪钳从两侧椎间隙向椎体中部逐渐咬除剩余椎体后壁。使用骨蜡及明胶海绵止血。前述步骤完成后椎体骨化物复合体与脊柱间的硬性连接即已断开。使用多把椎体钉起子同时拧紧需提拉节段的椎体钉,可观察到椎体逐渐前移至紧贴钛板。如未观察到椎体前移,需停止提拉,探查椎体骨化物复合体四周是否残留未断开的骨质并处理。仔细止血并冲洗切口,放置引流管,逐层关闭手术切口。
1.3 术后处理术后予抗炎、脱水、补液、营养神经、激素等常规处理,术后待引流量 < 10 mL/d时拔除引流管。术后使用头颈胸支具固定3个月,以保证术后早期活动及骨质融合。
1.4 疗效评价所有患者术前、术后均行标准颈椎正侧位X线、CT三维重建及MRI检查。在颈椎标准侧位X线片中,按照Boijsen法[5]测量颈椎椎管矢状径,即从椎体后缘中点到棘突根部最近点之间的距离。在横断面CT中选取骨化最严重的层面,测量骨化物横截面积、椎管横截面积,并计算椎管狭窄率。椎管狭窄率(%)=骨化物横截面积/椎管横截面积×100%。
采用日本骨科学会(JOA)评分[6](17分法)、疼痛视觉模拟量表(VAS)评分[7]评价患者神经功能和疼痛情况。用JOA评分改善率作为疗效评价指标,JOA评分改善率(%)=(术后JOA评分-术前JOA评分)/(17-术前JOA评分)×100%;JOA评分改善率≥80%为优,≥50%且 < 80%为良,≥5%且 < 50%为可,< 5%为差。
1.5 统计学处理应用SPSS 21.0软件对数据进行统计学分析。计量资料以x±s表示,术前、末次随访数据采用配对t检验,以P < 0.05为差异有统计学意义。
2 结果所有患者手术顺利完成。所有患者随访3~6个月,平均4.2个月。术后患者目标椎体明显前移,椎管横截面积由术前的(119.1±48.3)mm2增加为术后的(215.6±62.2)mm2,椎管狭窄率由术前的(60±10)%降低为(17±12)%,脊髓压迫解除。末次随访时各节段椎管矢状径均比术前增加,差异具有统计学意义(P < 0.05,表 1)。末次随访时,患者VAS评分从(9.38±2.70)分改善至(4.15±1.79)分,JOA评分从(14.15±1.99)分改善至(1.61±1.73)分,差异均有统计学意义(P < 0.05)。JOA评分改善率为(67.7±19.6)%,其中优4例,良7例,可2例,优良率为84.6%。
|
|
表 1 椎管矢状径 Table 1 Canal sagittal diameter |
13例患者中有1例出现脑脊液漏,嘱患者严格去枕平卧,予以抗生素预防感染,并行腰大池引流术,2周后痊愈。1例患者出现术后轴性疼痛,予以热敷并口服非甾体类抗炎药治疗,1周后症状明显缓解。所有患者均未出现术后血肿、切口感染、食管瘘、深部感染、血管损伤或术中脊髓损伤等严重并发症。典型病例影像学资料见图 1。
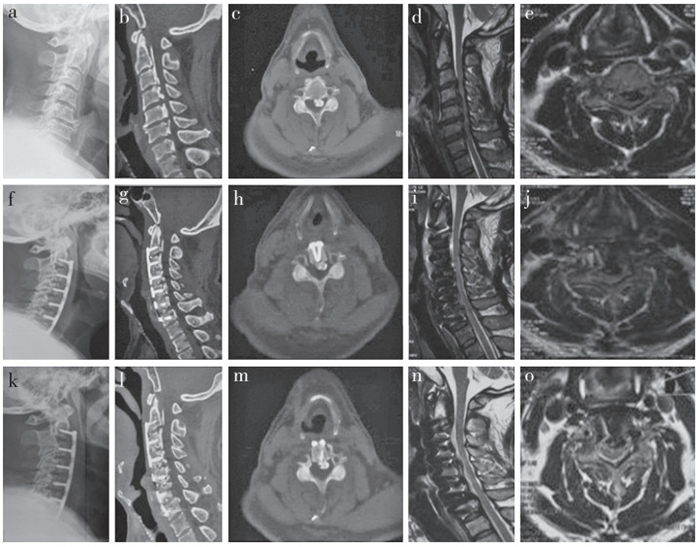
|
a:术前侧位X线片示颈椎严重退变,生理曲度消失 b,c:术前CT示C2~7 OPLL,椎管矢状径减小,椎管横截面积减小 d,e:术前MRI示C3~7椎管严重狭窄,脊髓严重受压,脑脊液带消失,局部脊髓高信号改变 f:术后侧位X线片示C3~6椎体前移,生理曲度恢复 g,h:术后CT示椎体及骨化物前移,椎管矢状径明显扩大,椎管横截面积明显增大,椎管狭窄率降低 i,j:术后MRI示椎管横截面积明显增大,脊髓压迫解除,脑脊液带恢复,脊髓形态恢复正常 k~o:术后3个月影像学资料示颈椎生理曲度恢复,序列稳定,两侧开槽区域融合良好,椎管矢状径和横截面积明显增大,脊髓压迫解除,脑脊液带恢复,脊髓形态恢复正常 a:Preoperative lateral roentgenograph shows serious cervical degeneration and poor physiological curvature b, c:Preoperative CTs show C2-7 OPLL, reduction of canal sagittal diameter and cross-sectional area d, e:Preoperative MRIs show C3-7 severe spinal cord compression, disappearance of cerebrospinal fluid band, high signal change of local spinal cord f:Postoperative lateral roentgenograph shows C3-6 anterior displacement, and recovery of physiological curvature g, h:Postoperative sagittal CTs show anterior displacement of vertebral body and ossification, and enlargement of canal sagittal diameter and cross-sectional area, and decrease of stenosis rate i, j:Postoperative MRIs show enlargement of cross-sectional area, relief of spinal cord compression, recovery of cerebrospinal fluid band and normal spinal cord morphology k-o:Imaging data at postoperative 3 months show recovery of cervical curvature, stability of sequence, good fusion in both sides of slotting area, enlargement of canal sagittal diameter and cross-sectional area, relief of spinal cord compression, recovery of cerebrospinal fluid band and normal spinal cord morphology 图 1 典型病例影像学资料 Figure 1 Imaging data of a typical case |
颈椎OPLL是由于颈椎骨化的后纵韧带自前方压迫颈脊髓和神经根,产生肢体感觉和运动障碍及内脏植物神经功能紊乱的一种病因不明的渐进性疾病。Fujimori等[8]对1 500例日本患者的CT资料进行分析,发现颈椎OPLL总体发生率为6.3%,男性8.3%,女性3.4%。
颈椎OPLL一般病情进展缓慢,早期可无明显临床症状。因此,当骨化发生到一定程度引起颈椎椎管狭窄压迫脊髓并引起临床症状时,骨化往往达了比较严重的程度。目前治疗颈椎OPLL的手术方式有颈椎前路椎体次全切除减压融合术(ACCF)、颈椎前路椎间盘切除融合术(ACDF)、颈椎后路椎板切除术、颈椎后路椎管扩大椎板成形术等。但对于严重的颈椎OPLL的术式选择,目前尚无统一认识。
在颈椎OPLL的前路治疗方案中,ACDF常难以完整切除骨化物,导致减压不彻底和术后骨化物继续发展并压迫脊髓[9]。因此,ACCF被作为常用的治疗OPLL的前路手术方式。ACCF可通过直接切除骨化物达到直接减压脊髓的目的,手术效果维持时间相对较长[10],术后神经功能恶化的发生率 < 1%[11]。Wang等[12]的一项包含123例行ACCF和216例行颈椎后路减压术患者的Meta分析表明行前路手术患者的结局指标和功能恢复指标均优于行后路手术的患者。对于存在严重颈椎后凸畸形的OPLL患者,前路手术也是首选的治疗方案[13]。
ACCF和ACDF可直接切除骨化物,对脊髓进行直接减压,但对技术要求较高,术后并发症多,如脑脊液漏、术后神经功能恶化等[14]。颈椎后路椎板切除术和椎管扩大椎板成形术可通过脊髓后移达到间接减压的目的,然而有相关文献报道后路手术术后症状可能加重需再手术,以及骨化物持续进展及后凸畸形的发生,也容易导致轴性疼痛、C5神经根麻痹等并发症的发生[15-18]。Khuyagbaatar等[19]认为颈椎后路椎板切除术后应力高度集中在神经根可能是导致C5神经根麻痹的主要原因,因为韧带骨化增加了脊髓的移位和神经根的移位,后凸畸形也会导致术后神经根压力的增加。
Yoshii等[20]通过一项多中心回顾研究发现,在OPLL患者的治疗中,前路手术和后路手术的术后恢复率相似,但在伴有颈椎后凸畸形的OPLL患者中,前路手术的神经功能恢复率明显优于后路。此外,前路手术的术后颈部疼痛较轻,但围手术期并发症的发生率更高。
目前对颈椎OPLL的治疗现状是前路手术具有较好的手术效果,但也伴随着较高的并发症发生率及较大的手术操作难度,后路手术具有较低的并发症发生率但手术效果欠佳[20-22]。本院史建刚团队[3]提出一种新的颈椎前路手术方式——ACAF,治疗颈椎OPLL,不直接切除骨化物即可获得脊髓直接减压并防止脊髓后移的手术效果,集合了目前颈椎前路手术直接减压和后路手术安全性高的优点。
ACAF通过颈椎椎体及骨化物的整体前移,直接扩大椎管矢状径及椎管横截面积,有效降低椎管狭窄率,同时可以防止脊髓后移,达到脊髓减压的目的。相较于ACCF和ACDF,ACAF可对长节段的连续型和混合型OPLL进行椎体前移获得脊髓直接减压的效果。一般认为ACCF的骨化物安全有效的切除范围为12.0~14.0 mm,对于更大范围或宽基底的骨化物,ACCF常常难以直接切除;ACAF的两侧开槽宽度及椎间隙的减压宽度达到18.0~20.0 mm,超过脊髓的解剖宽度[23],可同时对脊髓和神经根进行彻底减压。ACCF直接切除骨化物时,无论是分块切除还是“漂浮法”,均可能对脊髓造成直接损伤,其发生率高达14%[24];ACAF不直接切除骨化物,最大限度避免了对脊髓的损伤,增加了手术的安全性。
因ACAF开槽宽度较大,为避免损伤椎动脉,ACAF术中使用钩椎关节作为其解剖标志,因解剖学研究证明,钩椎关节在脊髓外侧横突孔内侧,与横突孔相距 > 0.5 cm[25],具有减压的有效性和避免椎动脉损伤的安全性。有文献报道,钩椎关节顶端和横突孔之间的距离在C2~4水平为3.0 mm,在C5~7水平为1.5 mm。术中一旦遇到钩椎关节顶端,则预示距离横突孔内侧缘的距离 < 3.0 mm[26]。开槽时除注意开槽宽度外,还需注意开槽方向,方向错误易导致靠外或靠内。由于颈椎前路手术采用单侧气管食管与颈动脉鞘间隙入路,术者站在患者的一侧进行操作,故向深部开槽时常不自主地向对侧偏斜,在使用头戴放大镜的医师中尤易出现,这个问题在ACCF术中也同样存在。ACAF术中如开槽位置正确,但开槽方向出现偏斜,则导致术者侧开槽宽度不足碰到OPLL,对侧开槽过宽碰到椎弓根。故开槽时除正确选择开槽位置外,一定要注意垂直开槽。ACAF术中应从颈长肌前方正中切开颈前筋膜,用电刀及锐利的神经剥离子由骨膜下向外侧剥离颈长肌至钩椎关节外缘,将走行于颈长肌和椎前筋膜浅面的交感干一同向外牵开以作保护[27],在牵拉颈动脉鞘时尽量避免下压拉钩损伤颈交感干[28]。
本研究中,有2例患者曾行颈椎后路椎板切除术,1例患者曾行颈椎后路半椎板切除内固定术。此3例患者在后路手术术后神经症状均无明显改善,并随时间进展而逐步加重,后于本院行ACAF进行翻修,术后患者神经功能均改善明显。颈椎后路手术由于不能做到直接减压,加之术后脊髓向后漂移,导致神经功能改善欠佳[20],ACAF将椎体及骨化物整体前移,对脊髓直接减压,恢复有效的椎管容积及脑脊液带,有效改善术后神经功能情况。
综上所述,ACAF结合了传统颈椎前路手术直接减压和颈椎后路手术操作安全的特点,有效扩大了颈椎椎管矢状径及椎管横截面积,降低了椎管狭窄率,彻底减压脊髓,短期随访疗效良好,可作为治疗颈椎OPLL的选择方案。但由于本研究临床病例数较少、随访时间较短,其适应证的选择、并发症的预防及远期疗效尚需进一步大样本长期随访研究来证实,同时,仍需与行传统颈椎前路或后路手术患者的对比研究来证实ACAF的优缺点。
| [1] | Chen Z, Liu B, Dong J, et al. Comparison of anterior corpectomy and fusion versus laminoplasty for the treatment of cervical ossification of posterior longitudinal ligament:a meta-analysis[J]. Neurosurg Focus, 2016, 40(6): E8. DOI:10.3171/2016.3.FOCUS15596 |
| [2] | 贾连顺. 颈椎病的现代概念[J]. 脊柱外科杂志, 2004, 2(2): 123–126. |
| [3] | 孙璟川, 史建刚, 王元, 等. 颈椎前路椎体骨化物复合体前移融合术治疗严重颈椎后纵韧带骨化症[J]. 第二军医大学学报, 2017, 38(8): 1053–1059. |
| [4] | Sun J, Shi J, Xu X, et al. Anterior controllable antidisplacement and fusion surgery for the treatment of multilevel severe ossification of the posterior longitudinal ligament with myelopathy:preliminary clinical results of a novel technique[J]. Eur Spine J, 2017, 28. DOI:10.1007/s00586-017-5437-4 |
| [5] | Boijsen E. The cervical spinal canal in intraspinal expansive processes[J]. Acta radiol, 1954, 42(2): 101–115. DOI:10.3109/00016925409175101 |
| [6] | Fukui M, Chiba K, Kawakami M, et al. Japanese Orthopaedic Association cervical myelopathy evaluation questionnaire(JOACMEQ):Part 2. Endorsement of the alternative item[J]. J Orthop Sci, 2007, 12(3): 241–248. DOI:10.1007/s00776-007-1119-0 |
| [7] | Huskisson EC. Measurement of pain[J]. Lancet, 1974, 2(7889): 1127–1131. |
| [8] | Fujimori T, Watabe T, Iwamoto Y, et al. Prevalence, concomitance, and distribution of ossification of the spinal ligaments:results of whole spine CT scans in 1500 Japanese patients[J]. Spine(Phila Pa 1976), 2016, 41(21): 1668–1676. DOI:10.1097/BRS.0000000000001643 |
| [9] | Chen Y, Yang L, Liu Y, et al. Surgical results and prognostic factors of anterior cervical corpectomy and fusion for ossification of the posterior longitudinal ligament[J]. PLoS One, 2014, 9(7): e102008. DOI:10.1371/journal.pone.0102008 |
| [10] | Yang HS, Chen DY, Lu XH, et al. Choice of surgical approach for ossification of the posterior longitudinal ligament in combination with cervical disc hernia[J]. Eur Spine J, 2010, 19(3): 494–501. DOI:10.1007/s00586-009-1239-7 |
| [11] | Choi S, Lee SH, Lee JY, et al. Factors affecting prognosis of patients who underwent corpectomy and fusion for treatment of cervical ossification of the posterior longitudinal ligament:analysis of 47 patients[J]. J Spinal Disord Tech, 2005, 18(4): 309–314. DOI:10.1097/01.bsd.0000161236.94894.fc |
| [12] | Wang S, Xiang Y, Wang X, et al. Anterior corpectomy comparing to posterior decompression surgery for the treatment of multi-level ossification of posterior longitudinal ligament:a meta-analysis[J]. Int J Surg, 2017, 40: 91–96. DOI:10.1016/j.ijsu.2017.02.058 |
| [13] | Saetia K, Cho D, Lee S, et al. Ossification of the posterior longitudinal ligament:a review[J]. Neurosurg Focus, 2011, 30(3): E14. DOI:10.3171/2011.1.FOCUS10262 |
| [14] | Lei T, Shen Y, Wang LF, et al. Cerebrospinal fluid leakage during anterior approach cervical spine surgery for severe ossification of the posterior longitudinal ligament:prevention and treatment[J]. Orthop Surg, 2012, 4(4): 247–252. DOI:10.1111/os.2012.4.issue-4 |
| [15] | Odate S, Shikata J, Soeda T, et al. Surgical results and complications of anterior decompression and fusion as a revision surgery after initial posterior surgery for cervical myelopathy due to ossification of the posterior longitudinal ligament[J]. J Neurosurg Spine, 2017, 26(4): 466–473. DOI:10.3171/2016.9.SPINE16430 |
| [16] | Shibuya S, Komatsubara S, Oka S, et al. Differences between subtotal corpectomy and laminoplasty for cervical spondylotic myelopathy[J]. Spinal Cord, 2010, 48(3): 214–220. DOI:10.1038/sc.2009.114 |
| [17] | Tokuhashi Y, Ajiro Y, Umezawa N. A patient with two re-surgeries for delayed myelopathy due to progression of ossification of the posterior longitudinal ligaments after cervical laminoplasty[J]. Spine(Phila Pa 1976), 2009, 34(2): E101–105. DOI:10.1097/BRS.0b013e31818a3135 |
| [18] | 福嘉欣, 江毅. 颈椎后路单开门椎管扩大成形术后相关并发症的研究进展[J]. 脊柱外科杂志, 2016, 14(1): 58–61. |
| [19] | Khuyagbaatar B, Kim K, Park WM, et al. Biomechanical investigation of post-operative C5 palsy due to ossification of the posterior longitudinal ligament in different types of cervical spinal alignment[J]. J Biomech, 2017, 57: 54–61. DOI:10.1016/j.jbiomech.2017.03.019 |
| [20] | Yoshii T, Sakai K, Hirai T, et al. Anterior decompression with fusion versus posterior decompression with fusion for massive cervical ossification of the posterior longitudinal ligament with a ≥ 50% canal occupying ratio:a multicenter retrospective study[J]. Spine J, 2016, 16(11): 1351–1357. DOI:10.1016/j.spinee.2016.07.532 |
| [21] | Tani T, Ushida T, Ishida K, et al. Relative safety of anterior microsurgical decompression versus laminoplasty for cervical myelopathy with a massive ossified posterior longitudinal ligament[J]. Spine(Phila Pa 1976), 2002, 27(22): 2491–2498. DOI:10.1097/00007632-200211150-00013 |
| [22] | Iwasaki M, Kawaguchi Y, Kimura T, et al. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine:more than 10 years follow up[J]. J Neurosurg, 2002, 96(2 Suppl): 180–189. |
| [23] | Petty P. Surgical anatomy of the anterior cervical spine:the disc space, vertebral artery, and associated bony structures[J]. Neurosurgery, 1997, 41(1): 325. DOI:10.1097/00006123-199707000-00072 |
| [24] | Kim B, Yoon DH, Shin HC, et al. Surgical outcome and prognostic factors of anterior decompression and fusion for cervical compressive myelopathy due to ossification of the posterior longitudinal ligament[J]. Spine J, 2015, 15(5): 875–884. DOI:10.1016/j.spinee.2015.01.028 |
| [25] | Cardoso MJ, Koski TR, Ganju A, et al. Approach-related complications after decompression for cervical ossification of the posterior longitudinal ligament[J]. Neurosurg Focus, 2011, 30(3): E12. DOI:10.3171/2011.1.FOCUS10278 |
| [26] | Chan TY, Li X, Mak KC, et al. Normal values of cervical spinal cord diffusion tensor in young and middle-aged healthy Chinese[J]. Eur Spine J, 2015, 24(12): 2991–2998. DOI:10.1007/s00586-015-4144-2 |
| [27] | Bertrand MM, Alsaid B, Droupy S, et al. Anatomical basis of the coordination between smooth and striated urethral and anal sphincters:loops of regulation between inferior hypogastric plexus and pudendal nerve. Immuno-histological study with 3D reconstruction[J]. Surg Radiol Anat, 2016, 38(8): 963–972. DOI:10.1007/s00276-016-1655-4 |
| [28] | Aalto TJ, Leinonen V, Herno A, et al. Postoperative rehabilitation does not improve functional outcome in lumbar spinal stenosis:a prospective study with 2-year postoperative follow-up[J]. Eur Spine J, 2011, 20(8): 1331–1340. DOI:10.1007/s00586-011-1781-y |
 2018, Vol.16
2018, Vol.16  Issue(1): 8-13
Issue(1): 8-13


