2. 广东省中医院(广州中医药大学第二附属医院)脊柱骨科, 广州 510120
2. Department of Spinal Orthopaedics, Guangdong Provincial Hospital of Chinese Medicine(Second Affiliated Hospital of Guangzhou University of Chinese Medicine), Guangzhou 510120, Guangdong, China
腰椎融合术是治疗多种腰椎退行性疾病的标准手术方法,广泛用于治疗椎间盘退行性变、腰椎椎管狭窄、退行性腰椎滑脱及脊柱畸形等疾病。随着技术的进步,术后腰椎融合率亦逐步提高,但临床疗效却未取得相应进步。其中邻近节段退行性变(ASD)的发生是影响腰椎融合术后疗效的重要因素之一[1],它可导致患者腰腿部症状复发甚至需再次手术,近年来受到国内外学者的广泛关注。以往,ASD患者通常选择相应节段的后路单纯减压、减压并内固定延伸等,存在的问题主要包括原手术区域解剖层次不清晰、术区瘢痕粘连、损伤大、出血多、术后恢复时间长等[2]。斜外侧腰椎椎间融合术(OLIF)在2012年由Silvestre等[3]正式提出并广泛应用于临床,疗效满意。OLIF具有无需涉及椎管内减压,降低损伤神经根、破坏硬膜可能,无需破坏脊柱后方肌肉-韧带复合体、关节突关节等脊柱后方稳定结构,较大的植骨面有助于术后椎间融合等优点[4-7]。基于上述原因,本研究回顾性分析本院采用OLIF治疗的腰椎融合术后发生ASD患者的临床资料,探讨OLIF的应用价值。
1 资料与方法 1.1 一般资料纳入标准:①符合Min等[8]提出的腰椎融合术后ASD的诊断标准,即融合术后症状缓解 > 6个月,出现与ASD影像学改变一致的临床症状,采用OLIF治疗;②手术节段为L2 ~ 4;③轻度腰椎椎管狭窄症;④轻度(Ⅰ度、Ⅱ度)腰椎滑脱;⑤退行性腰椎侧后凸畸形;⑥随访资料完整。
2015年8月—2017年5月,按以上标准共纳入腰椎融合术后ASD患者11例,男8例、女3例,年龄56 ~ 78(66.5±8.7)岁;骨密度T值-1.7 ~ -3.3(-2.4±0.5)。所有患者均有不同程度腰腿部疼痛,伴/不伴下肢根性放射痛/麻木、感觉/运动功能受累。初次融合节段:L3/L4 1例、L4/L5 3例、L3/L4/L5 2例、L4/L5/S1 4例、L3/L4/L5/S1 1例。患者均在全麻下行腰椎后路融合椎弓根内固定术,术后临床症状改善理想,影像学提示减压充分、腰椎结构稳定。ASD发生在初次融合术后42 ~ 102(74.6±19.7)个月,位于融合节段上方,表现为腰椎椎管轻度狭窄6例、腰椎椎管轻度狭窄合并滑脱2例、腰椎椎管轻度狭窄合并腰椎椎间盘突出2例、腰椎椎间盘突出1例。
1.2 手术方法患者全身麻醉后,取右侧卧位,用C形臂X线机行腰椎正侧位透视,准确定位手术节段,体表标记目标椎间隙、肋骨下缘和髂嵴的位置。于手术节段椎间隙线与腋前线交点处做1个长约4 cm的切口,距椎间隙前缘约5 cm,切口方向与腹外斜肌肌纤维平行。将腹外斜肌、腹内斜肌、腹横肌沿其肌束纤维走向钝性分离到达腹膜后间隙,将腹膜及腹腔内容物移向前方,经腹膜后间隙用手指顺着后腹壁钝性分离腹膜、腰大肌及大血管鞘间隙,暴露腰大肌并将其向后牵拉,同时将交感神经及输尿管移向前侧(此时要注意避免过度牵拉腰大肌而损伤腰丛神经及腰大肌纤维,以免导致术后腰痛及神经痛),到达椎间盘层面。暴露目标椎间盘并将探针插入其中,C形臂X线机透视证实节段正确,使用扩张套筒序贯撑开,安装椎间撑开器及光源,充分显露手术节段椎间隙,取出套筒及导针。在相应节段椎间隙侧方切开纤维环,以刮匙和铰刀去除髓核组织及椎间隙上下终板组织,暴露软骨下骨,试模选择大小合适的Cage(枢法模,中国)并用同种异体骨充分填塞,可以用止血纱包绕Cage以免同种异体骨掉落,利用正交法原理垂直于椎间隙置入Cage。C形臂X线机透视观察Cage位置及大小合适,中线位于椎间隙正中,椎间隙及椎间孔高度增加后退出工作套管,用生理盐水冲洗术区,逐层缝合,关闭切口。部分患者视情况行后路经皮椎弓根螺钉内固定术。
1.3 术后处理术后常规应用抗生素48 h预防感染,术后第2天开始锻炼下肢肌肉,如抬高、等长收缩,常规摄腰椎正侧位X线片确认Cage位置,术后3 d佩戴腰围并在助行器辅助下离床活动,腰围佩戴时间约3个月。
1.4 评价指标记录手术时间、术中出血量、住院天数和手术相关并发症。分别于术后3、6个月及末次随访对患者进行门诊或电话随访。采用疼痛视觉模拟量表(VAS)评分[9]对患者腰腿痛进行评价,采用Oswestry功能障碍指数(ODI)[10]评价临床疗效。末次随访时由影像科专业技术人员摄腰椎标准正侧位、过伸过屈位X线片及CT观察椎间融合情况,手术节段活动度 < 4°和/或椎间隙有连续骨小梁形成判断为可靠融合。在X线片上测量椎间孔高度(IFH)、椎间隙背侧高度(DH)及腹侧高度(VH)。具体见图 1。

|
a:测量IFH b:测量椎间隙VH、DH a: Measurement of IFH b:Measurement of VH and DH of intervertebral space 图 1 测量指标示意图 Figure 1 Schematic diagram of measurement index |
采用SPSS 18.0软件对数据进行统计学分析,计量数据以x±s表示。手术前后ODI、VAS评分及影像学参数比较采用配对t检验;以P < 0.05为差异有统计学意义。
2 结果 2.1 一般情况所有病例均在OLIF微创管道下顺利完成手术,共13个手术节段,单节段9例,双节段2例;L2/L3 5例,L3/L4 4例,L2/L3/L4 2例;4例术中加行椎弓根螺钉内固定术。手术时间55 ~ 160(87±35)min,单节段手术时间55 ~ 77(64±8)min(不含内固定操作),术中出血量40 ~ 140(74.2±28.8)mL,住院5 ~ 11(6.7±1.9)d。
2.2 临床疗效11例患者随访6 ~ 27(17.7±6.8)个月。术后测量的椎间隙VH、DH均较术前分别增加(4.5±2.1)mm和(3.2±1.9)mm,IFH较术前增加(5.7±1.6)mm(表 1)。术后3 d、6个月及末次随访时腰腿痛VAS评分及ODI均较术前明显降低,差异均有统计学意义(P < 0.05,表 2)。至末次随访时,行椎弓根螺钉内固定的患者未见螺钉松动、移位或断裂;腰椎过伸过屈位X线片及部分患者三维重建CT示椎间融合良好。1例59岁男性患者术后3个月发生Cage下沉(术前骨密度T值为-2.5,单纯椎间融合),予以支具保护、抗骨质疏松治疗及密切随访,术后19个月CT检查示椎间融合,Cage未出现沉降或移位(图 2);其余10例患者均未出现Cage沉降或移位。
|
|
表 1 影像学测量指标 Table 1 Imaging measurement index |
|
|
表 2 VAS评分和ODI Table 2 VAS score and ODI |
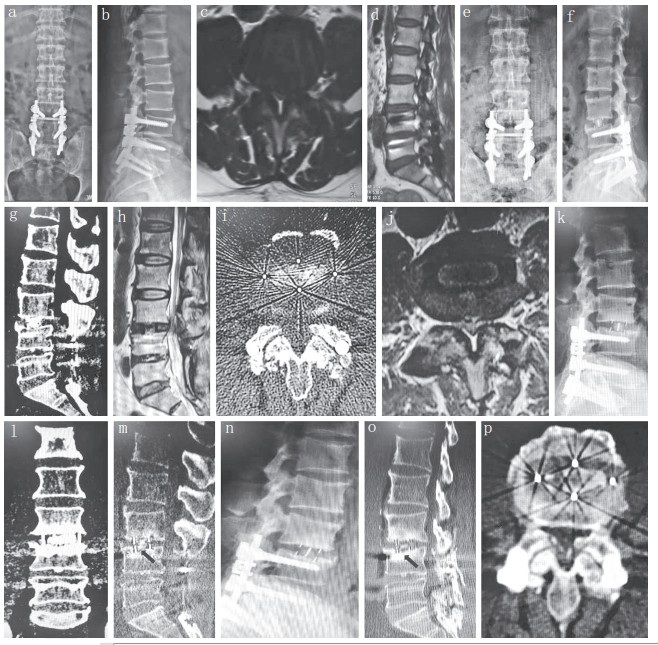
|
a ~ d:术前X线片及MRI示L3,4椎管狭窄 e,f:术后3 d X线片示椎间隙恢复 g ~ j:术后3 d CT及MRI示L3,4椎管面积增大,狭窄程度减轻,Cage位置良好 k ~ m:术后3个月X线片及CT示Cage沉降,未见移位 n ~ p:术后19个月X线片及CT示Cage无明显沉降,椎间骨性融合 a-d:Preoperative roentgenographs and MRIs show L3, 4 spinal canal stenosis e, f:Roentgenographs at postoperative 3 d show vertebral space recovery g-j:CTs and MRIs at postoperative 3 d show L3, 4 spinal canal area enlarged, stenosis degree reduced, and Cage in good position k-m:Roentgenograph and CTs at postoperative 3 months show Cage settlement without displacement n - p:Roentgenograph and CTs at postoperative 19 months show no significant progress in Cage settlement, and intervertebral bone fusion. 图 2 典型病例影像学资料 Figure 2 Radiologic data of a typical case |
所有患者术中均未发生重要神经、血管、输尿管及腹腔脏器损伤,无内置物相关并发症发生。2例(18.2%)患者术中透视时发现在椎间隙处理过程中损伤终板、Cage下沉,按照Malham等[11]提出的分型,均属于Ⅱ型沉降(冠状面上累及操作侧和对侧终板,矢状面上累及融合间隙尾端终板),术中联合后路椎弓根螺钉内固定,术后无相关临床症状,末次随访时未见融合器进一步下沉和移位。1例出现交感神经链损伤所致下肢症状,以下肢根性痛为主要表现,予以营养神经对症处理后缓解;1例出现一过性术侧腰大肌无力,屈髋肌力3+级,对症处理后4 d肌力恢复正常;1例发生腹膜撕裂,术中请胃肠外科专家行急诊手术修补腹膜。
3 讨论ASD一般是指脊柱融合术后融合节段上方或下方椎间盘以及椎间小关节等发生退行性变,包括头端椎体退行性变和尾端椎体退行性变。Park等[12]通过荟萃分析发现,影像学上ASD发生率为8% ~ 100%,临床症状ASD发生率为5.2% ~ 18.5%。Nakashima等[13]对215例行后路腰椎椎间融合术(PLIF)治疗的患者平均随访6.7年,认为术后5年和10年有临床症状的ASD发生率分别为16.5%、36.1%,每年以3.9%的速度递增。Zhang等[14]系统分析了4 206例腰椎融合术后患者资料,指出有影像学改变的ASD年发生率为5.9%,有临床症状的ASD年发生率为1.8%,融合节段越多,ASD发生率越高。Sears等[15]随访了1 000例行腰椎融合术的患者,平均随访63(5 ~ 192)个月,共有130例(13%)需再次手术治疗,ASD的年发生率为2.5%,单节段融合术后ASD年发生率为1.7%,双节段为3.6%,多节段(≥3)为5.0%,行椎板切除减压后ASD年发生率是未行椎板减压者的2.4倍。
ASD的发生是随年龄增长的自然过程,还是腰椎融合术所致,一直存在争议。Mannion等[16]认为发生ASD的3个主要原因是邻近椎间盘自身发生退行性变,融合术后引起邻近节段生物力学改变及手术对邻近解剖结构的破坏。文献[2, 14, 17-18]指出,ASD的主要影响因素包括个体因素(年龄、性别、工作性质、骨质疏松以及术前邻近节段退行性变情况)、手术因素(融合节段长短、手术方式)、内固定器的使用、矢状面平衡、椎间隙过度撑开等。生物力学实验表明,脊柱融合术尤其是内固定融合术后,相邻未融合节段的应力、位移增加,尤其是腰椎小关节,继发腰椎不稳和退行性变而再次出现症状,有临床症状的ASD患者非手术治疗无效时常需再次手术治疗[19]。
ASD患者行翻修手术,通常采用后路单纯椎管减压或椎管减压并融合内固定。Miwa等[20]回顾性分析18例PLIF术后因ASD再次行PLIF翻修的患者,JOA评分从7.7分提高至术后11.4分,17例(94%)患者认为术后效果优良,至末次随访时,10例(56%)认为效果优或好,2例因复发性ASD而接受第3次手术;作者据此认为,PLIF应用于ASD短期内疗效是满意的。但应用传统后路手术进行ASD翻修存在一定风险,由于邻近节段瘢痕形成,翻修时存在解剖层次不清晰、减压困难、术中易损伤神经根等弊端,出血多,术后切口部位疼痛的发生率相对较高,恢复时间长,相关费用也较高[21]。后路翻修手术会暴露上次椎板切除后形成的瘢痕组织,发生脑脊液漏的风险也相应增加[22]。Wang等[23]报道了1例采用前路腰椎椎间融合术(ALIF)处理的ASD患者(L5/S1),认为在严格把握适应证的基础上,ALIF可成为处理ASD的一种手术方式。ALIF能恢复椎间隙高度和生理性前凸,置入的融合器植骨面积大,融合率高,不对后方关节突关节形成侵扰[24]。但是,ALIF存在损伤髂血管、腹膜、腹腔内容物、输尿管及交感神经丛的可能,一旦发生可能造成严重后果;因此选择ALIF处理腰椎ASD时需要慎重。经椎间孔腰椎椎间融合术(TLIF)无需打开椎管即可完成神经根及椎管内减压,保留健侧关节突关节,有助于维持脊柱术后稳定性及减少内固定断裂的可能,但会破坏患侧关节突关节,更依赖内固定器的坚固性及稳定性,且椎体间Cage的接触面积较PLIF、ALIF少[25]。
OLIF从腹膜后间隙进入,无需游离血管、神经,无需进入椎管,可有效避免常规前路及后路手术的风险,创伤小,Cage植骨面积大,术后融合率高,该术式保留了前后方韧带的张力带作用,维持了脊柱稳定性[3]。此外,该术式不对后方小关节形成侵扰,理论上再发生ASD的风险也相对降低。有学者认为脊柱畸形、退行性滑脱及椎间盘疾病均是OLIF的适应证[7, 26],Wang等[23]指出,OLIF适用于腰椎术后ASD及同节段再次手术,因其能避免再次手术时打开原术口瘢痕组织,降低因瘢痕增生、组织结构不清晰而误伤神经根的可能。文献报道OLIF术后椎间隙DH可增加42% ~ 89%、椎间孔面积可增加25% ~ 66%、椎管面积可增加30% ~ 43%[27]。Sato等[4]对20例腰椎滑脱患者采用OLIF手术进行间接减压,术后疗效满意,椎间隙高度增加61%。本研究中11例患者术后腰腿痛VAS评分及ODI均显著改善,术后影像学资料评估示腰椎即刻稳定性佳,术后椎间隙DH、VH及IFH均较术前显著增加;表明OLIF可有效改善椎间隙及椎间孔高度,有利于腰椎生理曲度的恢复,且住院时间短、术后恢复快,易被患者接受。
Davis等[28]尝试在尸体上通过经斜侧方腹膜后入路到达椎间盘水平,发现可避开许多解剖上的重要结构,提出可避免前后路及侧方经腰大肌入路对各种相关组织的破坏所引发的并发症。Mehren等[7]报道812例接受OLIF治疗的患者,仅30例(3.7%)术中及住院期间出现并发症,包括5例术口感染、11例血肿形成、2例麻痹性肠梗阻、2例髂总静脉损伤、1例腹主动脉损伤及9例神经损伤,未见腹膜损伤;认为OLIF术后并发症发生率较其他入路手术低。
本研究中11例患者术中均未发生重要神经、血管、输尿管及腹腔脏器损伤,术后未见深部及表浅感染、血肿形成。神经损伤是腰椎融合术的常见手术并发症之一,由于OLIF需要通过牵拉腰大肌暴露工作通道,容易损伤腰丛神经、生殖股神经及交感神经链,导致下肢根性痛、感觉异常、腰大肌及腹股沟区感觉麻木、无力,甚至逆行性射精。Lykissas等[29]对451例行侧方入路腰椎椎间融合术的患者进行平均18个月的随访,出现持续性下肢根性活动障碍及感觉减退(分别为2.3%和9.6%)。本研究仅有1例术后出现下肢根性症状,1例出现一过性腰大肌无力,在术后3个月随访时根性症状及腰丛神经刺激症状均消失,考虑可能是椎间隙撑开后神经根从原本曲屈受压状态改为轻度伸直牵拉状态,神经根敏感紧张易出现根性症状,给予神经营养、抗感染及止痛药物,充分休息后神经根适应此状态后症状缓解。
通过侧方进行Cage置入较后路可以提供更大的植骨面积,但在彻底骨融合前,仍存在Cage下沉、移位等可能。Le等[30]报道140例行腰椎侧方融合手术的患者,14.3%术后随访出现不同程度的Cage下沉或移位,作者认为此现象与术中终板损伤有关,且发生率与融合节段数呈正相关,单节段融合Cage下沉发生率为10.3%,4节段融合Cage下沉发生率高达50.0%;Cage下沉亦与Cage宽度呈正相关,22 mm宽的Cage术后下沉发生率为1.9%,而18 mm宽的Cage术后下沉率高达14.1%。本研究中1例患者术后出现无症状Cage下沉,术后3、6、9个月复查随访示Cage下沉后未移位,结合患者骨密度T值为-2.5,提示骨质疏松,遂予以支具保护、抗骨质疏松治疗及密切随访,至末次随访时Cage未再继续下沉且椎间骨性融合。因此,建议术前常规对患者进行骨密度检查,合并严重骨质疏松症的患者(T值≤-2.5)不建议行OLIF治疗,严重骨质疏松症患者置入Cage后容易下沉,导致神经根管因椎间隙塌陷未能充分减压而影响减压效果。
综上所述,OLIF治疗腰椎融合术后ASD的早期临床疗效满意,并发症少,是一种安全有效的手术方式。但本研究还存在病例数少,随访时间短,缺乏对照研究等不足,后期还需要更多病例以及中、长期随访的病例对照研究来进一步证实。
| [1] | Helgeson MD, Bevevino AJ, Hilibrand AS. Update on the evidence for adjacent segment degeneration and disease[J]. Spine J, 2013, 13(3): 342–351. DOI:10.1016/j.spinee.2012.12.009 |
| [2] | Scemama C, Magrino B, Gillet P, et al. Risk of adjacentsegment disease requiring surgery after short lumbar fusion:results of the French Spine Surgery Society Series[J]. J Neurosurg Spine, 2016, 25(1): 46–51. |
| [3] | Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion:oblique lumbar interbody fusion in 179 patients[J]. Asian Spine J, 2012, 6(2): 89–97. DOI:10.4184/asj.2012.6.2.89 |
| [4] | Sato J, Ohtori S, Orita S, et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion:oblique lateral interbody fusion for degenerated lumbar spondylolisthesis[J]. Eur Spine J, 2017, 26(3): 671–678. DOI:10.1007/s00586-015-4170-0 |
| [5] | Abe K, Orita S, Mannoji C, et al. Perioperative complications in 155 patients who underwent oblique lateral interbody fusion surgery[J]. Spine(Phila Pa 1976), 2017, 42(1): 55–62. DOI:10.1097/BRS.0000000000001650 |
| [6] | Li JX, Phan K, Mobbs R. Oblique lumbar interbody fusion:technical aspects, operative outcomes, and complications[J]. World Neurosurg, 2017, 98: 113–123. DOI:10.1016/j.wneu.2016.10.074 |
| [7] | Mehren C, Mayer HM, Zandanell C, et al. The oblique anterolateral approach to the lumbar spine provides access to the lumbar spine with few early complication[J]. Clin Orthop Relat Res, 2016, 474(9): 2020–2027. DOI:10.1007/s11999-016-4883-3 |
| [8] | Min JH, Jang JS, Jung B, et al. The clinical characteristics and risk factors for the adjacent segment degeneration in instrumented lumbar fusion[J]. J Spinal Disord Tech, 2008, 21(5): 305–309. DOI:10.1097/BSD.0b013e318142b960 |
| [9] | Huskisson EC. Measurement of pain[J]. Lancet, 1974, 2(7889): 1127–1131. |
| [10] | Fairbank JC, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire[J]. Physiotherapy, 1980, 66(8): 271–273. |
| [11] | Malham GM, Parker RM, Blecher CM, et al. Assessment and classification of subsidence after lateral interbody fu sion using serial computed tomography[J]. J Neurosurg Spine, 2015, 23(4): 589–597. |
| [12] | Park P, Garton HJ, Gala VC, et al. Adjacent segment disease after lumbar or lumbosacral fusion:review of the literature[J]. Spine (Phila Pa 1976), 2004, 29(17): 1938–1944. DOI:10.1097/01.brs.0000137069.88904.03 |
| [13] | Nakashima H, Kawakami N, Tsuji T, et al. Adjacent segment disease after posterior lumbar interbody fusion:based on cases with a minimum of 10 years of follow-up[J]. Spine (Phila Pa 1976), 2015, 40(14): E831–E841. DOI:10.1097/BRS.0000000000000917 |
| [14] | Zhang C, Berven SH, Fortin M, et al. Adjacent segment degeneration versus disease after lumbar spine fusion for degenerative pathology:a systematic review with metaanalysis of the literature[J]. Clin Spine Surg, 2016, 29(1): 21–29. DOI:10.1097/BSD.0000000000000328 |
| [15] | Sears WR, Sergides IG, Kazemi N, et al. Incidence and prevalence of surgery at segments adjacent to a previous posterior lumbar arthrodesis[J]. Spine J, 2011, 11(1): 11–20. |
| [16] | Mannion AF, Leivseth G, Brox JI, et al. ISSLS prize winner:long-term follow-up suggests spinal fusion is associated with increased adjacent segment disc degeneration but without influence on clinical outcome:results of a combined follow-up from 4 randomized controlled trials[J]. Spine (Phila Pa 1976), 2014, 39(17): 1373–1383. DOI:10.1097/BRS.0000000000000437 |
| [17] | Saavedra-Pozo FM, Deusdara RA, Benzel EC. Adjacent segment disease perspective and review of the literature[J]. Ochsner J, 2014, 14(1): 78–83. |
| [18] | Radcliff KE, Kepler CK, Jakoi A, et al. Adjacent segment disease in the lumbar spine following different treatment interventions[J]. Spine J, 2013, 13(10): 1339–1349. DOI:10.1016/j.spinee.2013.03.020 |
| [19] | Hartmann F, Dietz SO, Kuhn S, et al. Biomechanical comparison of an interspinous device and a rigid stabilization on lumbar adjacent segment range of motion[J]. Acta Chir Orthop Traumatol Cech, 2011, 78(5): 404–409. |
| [20] | Miwa T, Sakaura H, Yamashita T, et al. Surgical outcomes of additional posterior lumbar interbody fusion for adjacent segment disease after single-level posterior lumbar interbody fusion[J]. Eur Spine J, 2013, 22(12): 2864–2868. DOI:10.1007/s00586-013-2863-9 |
| [21] | Parker SL, Shau DN, Mendenhall SK, et al. Factors influencing 2-year health care costs in patients undergoing revision lumbar fusion procedures[J]. J Neurosurg Spine, 2012, 16(4): 323–328. |
| [22] | Khan IS, Sonig A, Thakur JD, et al. Perioperative complications in patients undergoing open transforaminal lumbar interbody fusion as a revision surgery[J]. J Neurosurg Spine, 2013, 18(3): 260–264. |
| [23] | Wang MY, Vasudevan R, Mindea SA. Minimally invasive lateral interbody fusion for the treatment of rostral adjacent-segment lumbar degenerative stenosis without supplemental pedicle screw fixation[J]. J Neurosurg Spine, 2014, 21(6): 861–866. |
| [24] | Li J, Dumonski ML, Liu Q, et al. A multicenter study to evaluate the safety and efficacy of a stand-alone anterior carbon Ⅰ/F Cage for anterior lumbar interbody fusion:two-year results from a food and drug administration investigational device exemption clinical tria[J]. Spine (Phila Pa 1976), 2010, 35(26): E1564–E1570. DOI:10.1097/BRS.0b013e3181ef5c14 |
| [25] | Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion:techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF[J]. J Spine Surg, 2015, 1(1): 2–18. |
| [26] | Phan K, Maharaj M, Assem Y, et al. Review of early clinical results and complications associated with oblique lumbar interbody fusion(OLIF)[J]. J Clin Neurosci, 2016, 31: 23–29. DOI:10.1016/j.jocn.2016.02.030 |
| [27] | Malham GM, Parker RM, Goss B, et al. Indirect foraminal decompression is independent of metabolically active facet arthropathy in extreme lateral interbody fusion[J]. Spine(Phila Pa 1976), 2014, 39(22): 1303–1310. DOI:10.1097/BRS.0000000000000551 |
| [28] | Davis TT, Hynes RA, Fung DA, et al. Retroperitoneal oblique corridor to the L2-S1 intervertebral discs in the lateral position:an anatomic study[J]. J Neurosurg Spine, 2014, 21(5): 785–793. |
| [29] | Lykissas MG, Aichmair A, Hughes AP, et al. Nerve injury after lateral lumbar interbody fusion:a review of 919 treated levels with identification of risk factors[J]. Spine J, 2014, 14(5): 749–758. DOI:10.1016/j.spinee.2013.06.066 |
| [30] | Le TV, Baaj AA, Dakwar E, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion[J]. Spine (Phila Pa 1976), 2012, 37(14): 1268–1273. DOI:10.1097/BRS.0b013e3182458b2f |
 2019, Vol.17
2019, Vol.17  Issue(1): 18-24
Issue(1): 18-24


