2. 中国人民解放军联勤保障部队第904医院骨科, 无锡 214000;
3. 中国人民解放军南部战区总医院骨科, 广州 440104;
4. 中国人民解放军第960医院骨科, 济南 250000
2. Department of Orthopaedics, No. 904 Hospital of Joint Logistics Support Force of Chinese PLA, Wuxi 214000, Jiangsu, China;
3. Department of Orthopaedics, General Hospital of Southern Theater of Chinese PLA, Guangzhou 440104, Guangdong, China;
4. Department of Orthopaedics, No. 960 Hospital of Chinese PLA, Jinan 250000, Shandong, China
椎间盘造影术是将造影剂注入椎间盘,利用影像学手段观察椎间盘的形态,反映椎间盘的病理特征[1]。作为一种侵入性的有创诊断技术,椎间盘造影术一直存在争议[2]。然而,椎间盘造影术具有不可替代的优势,如典型的疼痛复制、直观地显示椎间盘损伤程度等[3],其临床应用仍十分广泛。近年来,椎间盘造影对椎间盘退行性变的影响倍受重视。临床研究表明,椎间盘造影可加速腰椎椎间盘退行性变的进展[4]。体内研究表明,椎间盘造影和椎间盘阻滞可显著增加椎间盘内细胞的凋亡[5]。体外细胞实验表明,局部麻醉药和非离子型造影剂对纤维环细胞和髓核细胞均有不利影响[6]。目前关于椎间盘造影术中注射造影剂剂量及注射速度仍没有明确的标准,且存在很大争议[7-9],本研究通过观察椎间盘造影过程中造影剂剂量对大鼠尾椎椎间盘退行性变的影响,为评估椎间盘造影术的临床价值和风险提供实验依据。
1 材料和方法 1.1 实验动物10只3月龄雄性SD大鼠(海军军医大学动物实验中心提供),体质量(350±50)g。选择3月龄的雄性SD大鼠是因为此时大鼠骨骼趋于成熟,以尽量减少年龄增长引起的实验误差[10]。每笼2只分笼饲养,环境温度(25±1)℃,12 h/12 h昼夜循环,自由饮食,实验前适应环境至少1周。所有动物实验均通过海军军医大学实验动物伦理委员会审批备案。
1.2 模型建立正常成人椎间盘内髓核容积约为3 mL,而临床中使用的常规椎间盘内注射剂量为2 mL。成年大鼠尾椎椎间盘的平均椎间盘高度为0.94 mm,髓核面积为3.30 mm2,总髓核体积为3.10 mm3[11]。因此,本研究选择2 μL模拟临床椎间盘造影中的常规注射剂量,选择3 μL即大鼠尾椎椎间盘的极限容积作为高剂量。
将实验大鼠用10%的水合氯醛以30 mL/kg的剂量麻醉。选取尾部4个椎间盘为研究对象:Co6/Co7为空白组,不行任何处理;Co7/Co8为对照组,以30号穿刺针刺入椎间盘但不进行注射;Co8/Co9为常规剂量组,以30号穿刺针注入2 μL碘海醇(欧乃派克,含碘质量浓度300 mg Ⅰ/mL,质量渗透摩尔量为780 mOsm/kg,上海通用电气药业有限公司);Co9/Co10为高剂量组,以30号穿刺针注入3 μL碘海醇。所有穿刺操作均在X线引导下完成,穿刺针方向平行于所选椎间隙的上下终板,以医用止血钳控制进入椎间盘的导针长度为5 mm(图 1a、b)。碘海醇的注入使用3 μL微量注射器(Hamilton,瑞士),注射完毕后将导针滞留于椎间盘内2 min以防止液体渗漏。

|
注:A ~ C,上位椎体前缘、中点和后缘高度;D ~ F,椎间隙前缘、中点和后缘高度;G ~ I,下位椎体前缘、中点和后缘高度。a:X线引导下进行椎间盘造影 b:椎间盘造影后的X线影像 c:DHI测量示意图 Note:A - C,height of anterior,middle and posterior edge of superior vertebra;D - F,height of anterior,middle and posterior edge of intervertebral space;G - I,height of anterior,middle and posterior edge of inferior vertebra. a: Discography guided by roentgenography b:Roentgenographic image after discography c:Schematic diagram of DHI measurement 图 1 X线引导下椎间盘造影及DHI测量 Fig. 1 Discography guided by roentogenography and DHI measurement |
椎间盘造影后2周及4周,使用X线检测椎间盘高度指数(DHI)的变化。将大鼠用浓度10%的水合氯醛以30 mL/kg的剂量经腹部注射麻醉,使大鼠尾部肌肉松弛以便观察。使用X线拍摄大鼠尾椎椎间盘高度。采用Han等[12]提出的方法测量DHI,即DHI(%)=2×(D+E+F)/(A+B+C+G+H+Ⅰ)×100%,其中A、B、C分别为上位椎体前缘、中点和后缘的高度,D、E、F分别为椎间隙前缘、中点和后缘的高度,G、H、Ⅰ分别为下位椎体前缘、中点和后缘的高度(图 1c)。所有图像均由2名对样本不知情的独立观察者测量。通过Sante DICOM Viewer FREE图像软件分析DHI的变化。
1.4 MRI检查椎间盘造影后2周及4周使用MRI对大鼠尾椎椎间盘信号进行检测。将大鼠用10%水合氯醛以30 mL/kg的剂量麻醉,使尾部肌肉松弛以便观察。采用3.0 T小动物MRI机(飞利浦,荷兰)对椎间盘进行薄层扫描,以T2加权像评估椎间盘信号改变情况[13]。所有测量均由2名医师各自独立进行,不同时间段椎间盘信号强度变化应用NIH Image软件进行分析。
1.5 椎间盘含水量的测定椎间盘造影4周后,随机取5只大鼠,将大鼠腹腔注射高浓度水合氯醛麻醉处死,分离椎间盘与周围软组织,然后迅速置于-20℃液氮中冷冻。10 min后取出冷冻的椎间盘,使用精度为0.01 mg的电子秤称得其湿质量(WW)。再将椎间盘置于65℃烘箱中脱水24 h,用同一台电子秤称得其干质量(DW)。每次称量均重复3次,取平均值。椎间盘含水量(%)=(WW-DW)/WW×100%。
1.6 组织学检查影像学数据采集完毕后,将另外5只大鼠腹腔注射高浓度水合氯醛麻醉处死,迅速取出Co6/Co7/Co8/Co9/Co10椎间盘组织,置于4%多聚甲醛溶液中固定24 h,用无菌蒸馏水冲洗后放入EDTA中脱钙48 h,经不同梯度乙醇脱水,二甲苯透明,石蜡包埋,沿平行于椎间盘软骨排列的方向进行5 μm连续切片,进行HE染色。使用HE组织学评分评估椎间盘退行性变的程度[14]。椎间盘退行性变组织学评分为0~6分。纤维环评分:0分,纤维环结构完整、无破损;1分,纤维环结构有破损,但小于整体纤维环的30%;2分,纤维环结构有破损,且大于整体纤维环的30%;3分,纤维环完全破损。髓核评分:0分,有典型的含有大空泡状结构的髓核细胞散在分布;1分,髓核细胞数量下降低于总细胞数的50%,存在或不存在细胞集聚;2分,髓核细胞数量中度至严重(> 50%)下降,且增殖性结缔组织占据小于50%总面积;3分,髓核中增殖性结缔组织占据大于50%总面积。
1.7 统计学处理使用SPSS 21.0软件对数据进行统计学分析,并使用Graphpad Prism 5软件作图。数据采用x±s表示,使用单因素方差分析比较组间差异,多重比较采用Student-Newman-Keuls检验;以P < 0.05为差异有统计学意义。
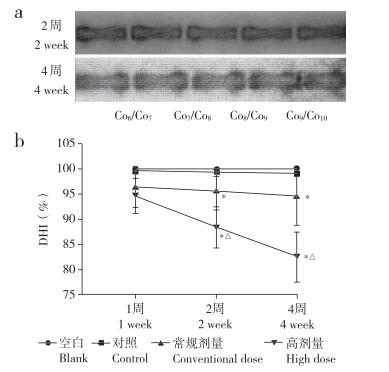
2 结果 2.1 造影剂剂量对椎间盘DHI的影响术后2周和4周时,与空白组相比,对照组DHI未见明显变化,常规剂量组和高剂量组DHI降低,且高剂量组比常规剂量组降低更为显著,差异均有统计学意义(P < 0.05,图 2)。
|
注:*与空白组相比,P < 0.05;△与常规剂量组相比,P < 0.05。a:椎间盘X线片b:各组DHI变化 Note:* P < 0.05,compared with blank group;△P < 0.05,compared with conventional dose group. a:Roentgenographic image of intervertebral disc b:Change of DHI in each group 图 2 X线检测造影剂剂量对椎间盘DHI的影响 Fig. 2 Effect of contrast agent dose on DHI of intervertebral disc detected by roentgenography |
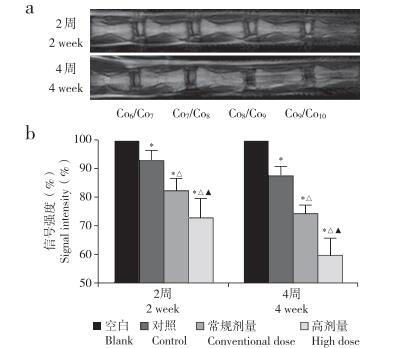
术后2周和4周时MRI检测示,与空白组相比,对照组、常规剂量组和高剂量组椎间盘信号均降低,且剂量越大信号越低,差异均有统计学意义(P < 0.05,图 3)。
|
注: *与空白组相比, P<0.05; △与对照组相比,P<0.05;▲与常 规剂量组相比,P<0.05。a:椎间盘MRIT2加权像b:椎间盘信号强度变化 Note: * P<0.05, compared with blank group;△P< 0.05, compared with control group;▲P<0.05, compared with conventional dose group. a: MRI T2-weighted image of intervertebral dise b: Signal intensity change of intervertebral disc 图 3 MRI检测造影剂剂量对椎间盘信号强度的影响 Fig. 3 Effect of contrast agent dose on signal intensity of intervertebral disc detected by MRI |
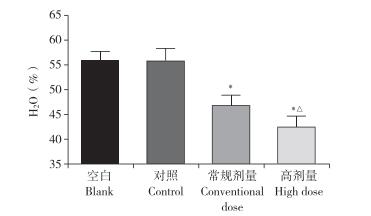
椎间盘造影术后4周,与空白组相比,对照组椎间盘含水量未见明显变化,常规剂量组和高剂量组椎间盘含水量降低,且高剂量组降低更为显著,差异均有统计学意义(P < 0.05,图 4)。
|
注:*与空白组相比,P < 0.05;△与常规剂量组相比,P < 0.05。 Note:* P < 0.05,compared with blank group;△P < 0.05,compared with conventional dose group. 图 4 造影剂剂量对椎间盘含水量的影响 Fig. 4 Effect of contrast agent dose on water content of intervertebral disc |
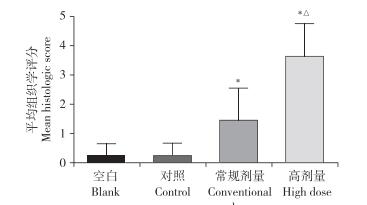
椎间盘造影术后4周,通过HE染色检测椎间盘组织学变化(图 5)。结果显示,空白组和对照组未见明显组织病理学改变,而常规剂量组和高剂量组椎间盘组织病理学改变明显,纤维环结构有破损,髓核细胞数量减少。病理组织学评分结果显示,常规剂量组和高剂量组椎间盘病理组织学评分高于空白组,且高剂量组病理组织学评分高于常规剂量组,差异均有统计学意义(P < 0.05,图 6)。

|
图 5 HE染色 Fig. 5 HE staining |
|
注:*与空白组相比,P < 0.05;△与常规剂量组相比,P < 0.05。 Note: * P < 0.05,compared with blank group;△ P < 0.05,compared with conventional dose group. 图 6 造影剂剂量对椎间盘组织学评分的影响 Fig. 6 Effect of contrast agent dose on histological score of intervertebral disc |
根据分子结构分类,造影剂可分为离子型和非离子型。离子型造影剂因其不良反应发生率高、肌体耐受性差而逐渐被非离子型造影剂所取代。非离子型造影剂也有一定的毒性,不同种类的非离子造影剂的细胞毒性不尽相同,可能与造影剂的渗透压和黏度相关[6, 15-17]。碘海醇属于第二代非离子型造影剂,目前在临床上应用最为广泛,也是临床椎间盘造影常用的造影剂[18]。体外细胞研究表明,碘海醇具有一定的细胞毒性[19]。本研究在大鼠体内就造影剂剂量对椎间盘退行性变的影响进行研究,于造影术后2周和4周分别对椎间盘的退行性变程度进行评估,评价指标包括DHI、MRI信号强度、椎间盘含水量和椎间盘组织学改变,证明了椎间盘造影中造影剂剂量对椎间盘退行性变的影响,为评估临床椎间盘造影技术的价值和风险提供了实验依据。
本研究证明,在动物体内碘海醇可以引起椎间盘退行性变,并且注射剂量越大,椎间盘退行性变越明显。椎间盘是人体最大的无血管组织,通过被动扩散完成营养和物质更新,椎间盘内存在特殊的高渗环境(430 ~ 496 mOsm/kg)[20-21]。本研究使用的碘海醇为上海通用电气药业有限公司产品,规格为300 mg Ⅰ/mL,渗透压为780 mOsm/kg,明显高于椎间盘的渗透压。且由于椎间盘是无血管器官,注射的碘海醇可以较长时间残存在椎间盘内,随着碘海醇注入量的增加,其对椎间盘的影响也较大。椎间盘退行性变的程度还和椎间盘的静水压有关。体内外研究已经清楚地表明,静水压可以显著影响细胞外基质成分、细胞合成代谢蛋白的活力和基因的表达[22-25]。碘海醇剂量对椎间盘退行性变的影响可能包括两方面原因:①在密闭的椎间盘内注射碘海醇体积越大,密闭椎间盘的静水压越高,导致椎间盘退行性变的程度越高;②碘海醇为高渗液体且具有细胞毒性,注射体积越大,高渗且有细胞毒性的溶液的量越大,椎间盘的退行性变程度也越大。此外,本研究还发现,仅进行穿刺针刺入而不进行注射的对照组MRI椎间盘信号也较未进行任何处理的空白组降低,说明针刺本身也可导致椎间盘退行性变。
本研究的不足之处包括以下几点。①仅从造影剂的剂量角度探讨椎间盘造影对椎间盘退行性变的影响,还有其他造成椎间盘退行性变的因素需要考虑,如穿刺针的直径、椎间盘尺寸(纤维环的厚度)、操作者的技术熟练程度、实验动物的营养状态及其他未知因素[26-27]。②研究对象为正常大鼠尾椎椎间盘,未研究椎间盘造影术对已经发生退行性变的椎间盘的影响,这和临床中椎间盘造影术的应用有所差异。主要基于两方面考虑:一方面,临床标准的椎间盘造影诊断技术需要1 ~ 2个邻近节段的椎间盘造影作为阴性对照,研究椎间盘造影术对正常椎间盘的影响可有效评估阴性对照的价值和风险;另一方面,考虑到椎间盘退行性变造模时,为避免模型的退行性变基线不尽相同造成系统误差,故使用正常椎间盘。③以大鼠椎间盘作为研究对象,大鼠尾椎椎间盘退行性变造模技术成熟,操作简便,安全性高,可重复性强,但大鼠椎间盘结构及髓核细胞形态、生理功能和人体有差异,且大鼠椎间盘内的渗透压和人体不尽相同,因此,碘海醇造成的大鼠椎间盘退行性变和人体实际情况存在差异。但因伦理等实际操作问题,本研究未将人体作为研究对象。造影剂剂量对人椎间盘退行性变的影响有待进一步深入研究。
综上所述,本研究发现,造影剂可引发椎间盘退行性变,且剂量越大,椎间盘退行性变的程度越高。因此,在进行椎间盘造影时,应当充分考虑造影剂剂量对椎间盘的影响,尽量减少造影剂的使用量,以使其对椎间盘的影响最小化。
| [1] |
Collis JS Jr, Gardner WJ. Lumbar discography. An analysis of one thousand cases[J]. J Neurosurg, 1962, 12: 452-461. |
| [2] |
Walsh TR, Weinstein JN, Spratt KF, et al. Lumbar discography in normal subjects. A controlled, prospective study[J]. J Bone Joint Surg Am, 1990, 72(7): 1081-1088. DOI:10.2106/00004623-199072070-00019 |
| [3] |
姜建元, 王洪立, 马昕, 等. 椎间盘造影在腰椎多节段退变性疾病诊治中的应用探讨[J]. 脊柱外科杂志, 2011, 9(4): 195-199. |
| [4] |
Carragee EJ, Don AS, Hurwitz EL, et al. 2009 ISSLS prize winner:does discography cause accelerated progression of degeneration changes in the lumbar disc:a ten-year matched cohort study[J]. Spine(Phila Pa 1976), 2009, 34(21): 2338-2345. DOI:10.1097/BRS.0b013e3181ab5432 |
| [5] |
Strube P, Pfitzner BM, Streitparth F, et al. In vivo effects of bupivacaine and gadobutrol on the intervertebral disc following discoblock and discography:a histological analysis[J]. Eur Radiol, 2017, 27(1): 149-156. DOI:10.1007/s00330-016-4358-7 |
| [6] |
Chee AV, Ren J, Lenart BA, et al. Cytotoxicity of local anesthetics and nonionic contrast agents on bovine intervertebral disc cells cultured in a three-dimensional culture system[J]. Spine J, 2014, 14(3): 491-498. DOI:10.1016/j.spinee.2013.06.095 |
| [7] |
Derby R, Lee SH, Lee JE, et al. Comparison of pressure-controlled provocation discography using automated versus manual syringe pump manometry in patients with chronic low back pain[J]. Pain Med, 2011, 12(1): 18-26. DOI:10.1111/j.1526-4637.2010.00990.x |
| [8] |
鲍达, 马远征, 白一冰, 等. 椎间盘压力测定在射频消融髓核成形术中的可行性研究[J]. 脊柱外科杂志, 2007, 5(5): 269-272. |
| [9] |
Kirberger RM, Cassel N, Carstens A, et al. The effects of repeated intravenous iohexol administration on renal function in healthy beagles—a preliminary report[J]. Acta Vet Scand, 2012, 54(1): 47. DOI:10.1186/1751-0147-54-47 |
| [10] |
Michalek AJ, Iatridis JC. Penetrating annulus fibrosus injuries affect dynamic compressive behaviors of the intervertebral disc via altered fluid flow:an analytical interpretation[J]. J Biomech Eng, 2011, 133(8): 084502. DOI:10.1115/1.4004915 |
| [11] |
Mao HJ, Chen QX, Han B, et al. The effect of injection volume on disc degeneration in a rat tail model[J]. Spine (Phila Pa 1976), 2011, 36(16): 1062-E1069. DOI:10.1097/BRS.0b013e3182027d42 |
| [12] |
Han B, Zhu K, Li FC, et al. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail[J]. Spine(Phila Pa 1976), 2008, 33(18): 1925-1934. DOI:10.1097/BRS.0b013e31817c64a9 |
| [13] |
Silveira JW, Issy AC, Castania VA, et al. Protective effects of cannabidiol on lesion-induced intervertebral disc degeneration[J]. PLoS One, 2014, 9(12): e113161. DOI:10.1371/journal.pone.0113161 |
| [14] |
Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture:correlation between the degree of disc injury and radiological and histological appearances of disc degeneration[J]. Spine(Phila Pa 1976), 2005, 30(1): 5-14. DOI:10.1097/01.brs.0000148152.04401.20 |
| [15] |
Duarte CG, Zhang J, Ellis S. Review of studies establishing the aging male spontaneously hypertensive rat as a detector and quantifier of the kidney toxicity of radiocontrast media and other chemicals[J]. Invest Radiol, 2001, 36(1): 56-63. DOI:10.1097/00004424-200101000-00008 |
| [16] |
Schick CS, Haller C. Comparative cytotoxicity of ionic and non-ionic radiocontrast agents on MDCK cell monolayers in vitro[J]. Nephrol Dial Transplant, 1999, 14(2): 342-347. DOI:10.1093/ndt/14.2.342 |
| [17] |
Lin Y, Li F, Chen W, et al. Single-level lumbar pyogenic spondylodiscitis treated with mini-open anterior debridement and fusion in combination with posterior percutaneous fixation via a modified anterior lumbar interbody fusion approach[J]. J Neurosurg Spine, 2015, 23(6): 747-753. DOI:10.3171/2015.5.SPINE14876 |
| [18] |
Yeganehkhah MR, Iranirad L, Dorri F, et al. Comparison between three supportive treatments for prevention of contrast-induced nephropathy in high-risk patients undergoing coronary angiography[J]. Saudi J Kidney Dis Transpl, 2014, 25(6): 117-1223. |
| [19] |
Heinrich M, Scheer M, Heckmann M, et al. Reversibility and time-dependency of contrast medium induced inhibition of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide(MTT) conversion in renal proximal tubular cells in vitro:comparison of a monomeric and a dimeric nonionic iodinated contrast medium[J]. Invest Radiol, 2007, 42(1): 732-738. |
| [20] |
Ishihara H, Warensjo K, Roberts S, et al. Proteoglycan synthesis in the intervertebral disk nucleus:the role of extracellular osmolality[J]. Am J Physiol, 1997, 272(5 Pt 1): C1499-C1506. |
| [21] |
van Dijk B, Potier E, Ito K. Culturing bovine nucleus pulposus explants by balancing medium osmolarity[J]. Tissue Eng Part C Methods, 2011, 17(11): 1089-1096. DOI:10.1089/ten.tec.2011.0215 |
| [22] |
Lotz JC, Colliou OK, Chin JR, et al. Compression-induced degeneration of the intervertebral disc:an in vivo mouse model and finite-element study[J]. Spine(Phila Pa 1976), 1998, 23(23): 2493-2506. DOI:10.1097/00007632-199812010-00004 |
| [23] |
Chen J, Yan W, Setton LA. Static compression induces zonal-specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro[J]. Matrix Biol, 2004, 22(7): 573-583. DOI:10.1016/j.matbio.2003.11.008 |
| [24] |
Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading[J]. Spine(Phila Pa 1976), 2000, 25(12): 1477-1483. DOI:10.1097/00007632-200006150-00005 |
| [25] |
Hutton WC, Elmer WA, Bryce LM, et al. Do the intervertebral disc cells respond to different levels of hydrostatic pressure?[J]. Clin Biomech(Bristol, Avon), 2001, 16(9): 782-734. |
| [26] |
Elliott DM, Yerramalli CS, Beckstein JC, et al. The effect of relative needle diameter in puncture and sham injection animal models of degeneration[J]. Spine(Phila Pa 1976), 2008, 33(6): 588-596. DOI:10.1097/BRS.0b013e318166e0a2 |
| [27] |
Issy AC, Castania V, Silveira JW, et al. Does a small size needle puncture cause intervertebral disc changes?[J]. Acta Cir Bras, 2015, 30(8): 574-579. DOI:10.1590/S0102-865020150080000009 |
 2020, Vol.18
2020, Vol.18  Issue(5): 325-330
Issue(5): 325-330


