20世纪80年代,Harms等[1]采用经椎间孔入路腰椎椎间融合术(TLIF)治疗腰椎退行性疾病,该术式相比后路腰椎椎间融合术(PLIF)更具优势,其可通过切除部分关节突关节从外侧暴露神经,从而减少神经根的牵拉,避免潜在的神经损伤。同时,TLIF还可通过最小化切除椎板、小关节来保持后柱的完整性。TLIF已被证实是一种安全有效的腰椎融合手术。随着脊柱外科理论与技术的进步,Foley等[2]在2003年首先提出利用肌肉间隙插入的管状牵开器行TLIF,即微创经椎间孔入路腰椎椎间融合术(MIS-TLIF),在多裂肌与最长肌间隙放置可撑开通道进行减压及融合,以期达到减少脊柱旁肌肉和软组织损伤的目的。既往研究[3]认为,传统开放TLIF与MIS-TLIF之间临床疗效无明显差异,也有部分学者认为MIS-TLIF优于传统TLIF,但既往研究均基于单节段腰椎退行性疾病。随着中国人口老龄化加剧,双节段腰椎椎间盘突出并不鲜见,一项长达20年的关于腰椎椎间盘突出症患者的中长期随访研究[4]显示,约有20%的患者为2个或以上节段的腰椎椎间盘突出。对于2个及以上节段的腰椎退行性疾病,MIS-TLIF是否仍存在优势仍值得商榷。基于此,本研究回顾性分析了2015年1月—2019年9月采用MIS-TLIF或传统TLIF治疗的56例双节段腰椎退行性疾病患者的临床资料,对临床疗效进行对比分析,现报告如下。
1 资料与方法 1.1 一般资料纳入标准:①具有腰椎椎管狭窄症的典型临床表现,如下肢神经症状、腰腿痛和/或间歇性跛行,且经规范非手术治疗3个月无效;②影像学检查明确为双节段椎间盘退行性变,如椎管狭窄,伴/不伴腰椎滑脱,且与临床症状、体征相符。排除标准:①严重脊柱侧凸、畸形、Ⅲ度及以上腰椎滑脱;②腰椎感染、肿瘤、严重骨质疏松症、运动神经元病等;③既往腰部手术或放射治疗史,病变节段广泛粘连。根据上述标准,纳入双节段腰椎退行性疾病患者56例,采用MIS-TLIF治疗26例(MIS-TLIF组)、采用传统开放TLIF治疗30例(TLIF组)。2组患者术前一般资料差异无统计学意义(P>0.05,表 1),具有可比性。所有手术均由同一高年资医师主刀完成,术中使用Quadrant微创操作系统(北京富乐科技开发有限公司,中国)。所有患者及家属在术前知晓具体手术方式和相关手术风险,并签署知情同意书。
|
|
表 1 2组患者一般资料 Tab. 1 General data of 2 groups |
TLIF组患者全身麻醉后取俯卧位,手术区域常规消毒、铺巾,以手术节段为中心做后正中切口,切开皮肤,沿棘突将椎旁肌与棘突、椎板、小关节囊分离,显露关节突关节,置入3枚椎弓根螺钉,咬除部分病灶节段关节突关节,凿除椎板,切除增生的黄韧带,显露硬膜囊及神经根,充分止血,摘除突出的髓核,切除椎间髓核,用铰刀清理椎间盘组织、刮除间隙上下软骨终板,椎间隙内植入修剪好的自体骨粒,斜向置入融合器,完成一侧减压后,根据患者术前症状及影像学表现决定是否行对侧减压。若需行对侧减压,则重复上述步骤,若无须对侧减压,于对侧置入椎弓根螺钉。于椎旁置入引流管1根,逐层缝合切口。
MIS-TLIF组患者全身麻醉后取俯卧位,手术区域常规消毒、铺巾,以手术节段为中心做后正中切口,切开皮肤,分离皮下,向两侧游离至筋膜层,于中线旁2 cm在最长肌与多裂肌间隙处切开筋膜,钝性分离肌间隙并置入导针,确定位置无误后,依次置入工作通道,其余步骤同TLIF组。
所有患者术后预防性使用抗生素72 h,定时观察双下肢活动情况及症状缓解情况,切口定期换药,拔除引流管后在支具保护下下床活动,支具佩戴至术后3个月。术后定期门诊随访并复查腰椎正侧位X线片及CT。
1.3 观察指标记录2组手术时间、术中透视次数、术中出血量、术后引流量、术后卧床时间、术后肌酸激酶(CK)等指标及并发症发生情况。术前及术后1周、3个月、12个月时采用疼痛视觉模拟量表(VAS)评分[5]和Oswestry功能障碍指数(ODI)[6]评估腰腿痛程度及腰椎功能。采用腰椎融合Bridwell分级[7]评估术后椎间融合情况。在术后腰椎CT上采用Rao分级[8]评价螺钉位置:A0型,未突破椎弓根内侧壁;A1型,椎弓根内侧壁突破 < 2 mm;A2型,椎弓根内侧壁突破≥2 mm且 < 4 mm。B0型,未突破椎弓根外侧壁;B1型,椎弓根外侧壁突破 < 2 mm;B2型,椎弓根外侧壁突破≥ 2 mm且 < 4 mm。
1.4 统计学处理采用SPSS 22.0软件对数据进行统计分析,符合正态分布的计量资料以x±s表示,组间比较采用t检验;计数资料以率或百分比表示,组间比较采用χ2检验;以P < 0.05为差异有统计学意义。
2 结果所有手术顺利完成,所有患者随访(14.7±2.1)个月。MIS-TLIF组较TLIF组手术时间长,术中透视次数多,但术后卧床时间短,差异均有统计学意义(P < 0.05,表 2);2组术中出血量、术后引流量及术后CK水平差异无统计学意义(P>0.05,表 2)。2组术后各时间点腰腿痛VAS评分及ODI均较术前显著改善,差异有统计学意义(P < 0.05,表 2),且随着随访时间延长呈进一步改善趋势;MIS-TLIF组术后1周腰痛VAS评分较TLIF组更低,差异有统计学意义(P < 0.05,表 2),2组术后3、12个月时腰腿痛VAS评分差异无统计学意义(P>0.05,表 2);各随访时间点2组间ODI差异无统计学意义(P>0.05,表 2)。
|
|
表 2 2组疗效评估指标比较 Tab. 2 Comparison of efficacy indexes of 2 groups |
2组患者均未发生内固定松动或融合器移位等并发症。术后12个月复查腰椎CT,2组患者均未发生不融合情况。根据Bridwell分级,MIS-TLIF组1级融合15例(57.7%),2级融合11例(42.3%);TLIF组1级融合17例(56.7%),2级融合13例(43.3%);椎间融合率组间比较差异无统计学意义(P>0.05)。
2组共置入椎弓根螺钉336枚。MIS-TLIF组置入180枚螺钉,其中A0型151枚,A1型23枚,A2型6枚;B0型107枚,B1型41枚,B2型32枚。TLIF组置入156枚螺钉,其中A0型124枚,A1型27枚,A2型5枚;B0型121枚,B1型31枚,B2型4枚。A型螺钉的分布组间差异无统计学意义(P>0.05);B型螺钉的分布组间差异有统计学意义(P < 0.05)。
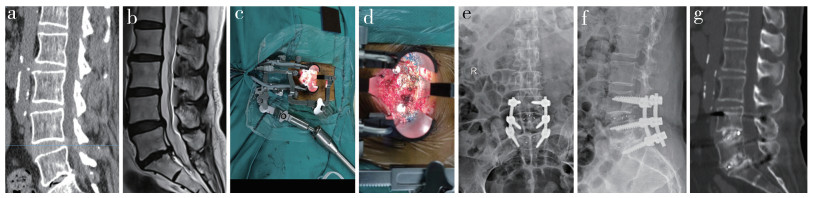
并发症发生情况:硬膜撕裂4例(MIS-TLIF组3例,TLIF组1例),给予手术切口加压包扎,适当补液,术后1周拔除引流管并严密缝合,伤口均愈合良好;术后下肢麻木加重4例(MIS-TLIF组3例,TLIF组1例),予抗炎、脱水、营养神经等对症处理后缓解;TLIF组切口愈合不良1例,予积极换药重新缝合后伤口愈合。2组并发症发生率差异无统计学意义(P>0.05)。MIS-TLIF组典型病例影像学资料见图 1。
|
图 1 MIS-TLIF组典型病例影像学资料 Fig. 1 Imaging data of a typical case in MIS-TLIF group 女,64岁,腰痛10年余,加重伴双下肢不适3个月 a、b:术前腰椎CT和MRI示L4/L5/S1椎间盘突出,L5/S1椎间盘退行性变,椎间隙变窄 c、d:术中放置工作通道 e、f:术后1个月腰椎正侧位X线片示内固定位置良好 g:术后3个月腰椎CT示椎间隙部分融合 Female, 64 years old, with lumbago for more than 10 years, aggravating with lower limb discomfort for 3 months a, b: Preoperative lumbar CT and MRI show L4/L5/S1 intervertebral disc herniation, L5/S1 intervertebral disc degeneration, and intervertebral space narrowing c, d: Intraoperative placement of working channels e, f: Anteroposterior and lateral roentgenographs at postoperative 1 month show good internal fixation position g: CT at postoperative 3 months shows partial fusion of intervertebral space |
TLIF的有效性与安全性已得到良好验证,de Kunder等[9]在一项荟萃分析中比较了PLIF与TLIF之间的优劣性,结果显示,TLIF在手术时间、术中出血量、并发症发生率等方面均优于PLIF。MIS-TLIF采用椎旁肌间隙入路,通过可扩张通道完成减压、椎间植骨融合,可保留椎旁肌及椎体后方结构的完整性,但其局限性在于通道限制了手术的操作空间,在手术过程中易损伤神经根及硬膜囊,对医师的操作技术及手术器械要求更高,学习曲线较为陡峭[10]。
多项研究发现,MIS-TLIF组手术时间长于传统TLIF组[11-13],术中透视频率高于传统TLIF组[14-15],而2种术式在VAS评分、ODI、JOA评分及术后融合率方面没有明显差别[14-21]。Arif等[22]的荟萃分析结果显示,MIS-TLIF较传统TLIF手术时间增加了126.3 min,透视时间增加了22.9 s;通过监测外科医师的双手、胸部、颈部和眼睛的暴露剂量发现,MIS-TLIF组的辐射剂量高于传统TLIF组30 µSv。本研究结果也显示,MIS-TLIF组的透视次数明显多于TLIF组,透视次数的增加延长了手术时间,同时增加了对医患的辐射暴露;2组末次随访时VAS评分、ODI、JOA评分及融合率差异均无统计学意义;且MIS-TLIF须双侧放置通道,其对椎旁肌肉的损伤并不少于传统TLIF,表现为术后2组CK水平无明显差异,且2组患者术后腰痛VAS评分相近亦证实了该结果。
本研究中MIS-TLIF组发生硬膜撕裂3例,TLIF组1例,可能是因为MIS-TLIF学习曲线陡峭,通道的限制导致手术视野狭小,操作空间受限,易撕裂硬膜囊。Lee等[23]的大样本量病例研究评估MIS-TLIF的学习曲线,认为医师至少要经过44例手术才能真正掌握该技术,才有可能缩短手术时间、减少透视次数,患者才能获得满意的临床疗效;同时,在学习过程中发生十二指肠破裂1例、融合器松动2例。Kang等[24]的研究统计了MIS-TLIF术中硬膜撕裂的情况,初次手术的4例患者中有1例发生硬膜撕裂,翻修手术的19例中有4例发生硬膜撕裂。Goertz等[25]发现,与体质量正常的患者相比,肥胖患者MIS-TLIF术中硬膜撕裂发生率更高。
本研究按照Rao分级对螺钉位置进行评价,结果显示,只有少数螺钉突破椎弓根内侧壁,MIS-TLIF组突破外侧壁的螺钉数量高于TLIF组。螺钉突破椎弓根外侧壁限制了螺钉长度,降低了把持力,同时可增加手术并发症的发生。既往也有研究[26-27]报道MIS-TLIF术中螺钉误置导致术后神经症状再次翻修的病例。Venier等[28]在MIS-TLIF术中使用CT导航引导置入408枚螺钉,根据Heary分类,置钉准确率为95.3%,19枚螺钉(4.7%)偏入椎管,但偏入距离均 < 2 mm,其认为通道下的狭窄空间限制了螺钉置入的准确性,提高了学习难度。Zhao等[29]的研究发现,MIS-TLIF术中上关节突损伤率为34.07%(62/182),通过logistics回归分析结果显示,体质量指数≥30 kg/m2及L5椎弓根螺钉置入是关节突关节损伤的独立危险因素。
综上所述,MIS-TLIF和传统TLIF治疗双节段腰椎退行性疾病的临床疗效相似,但MIS-TLIF在手术时间、术中射线暴露情况及椎旁肌肉损伤等方面并无明显优势,同时可能出现较高的术中并发症发生率及螺钉误置。因此,建议双节段腰椎退行性疾病患者选择传统TLIF治疗。本研究为单中心回顾性研究,纳入病例数量有限,且随访时间较短,2种术式的长期疗效尚待后续大样本量、长时间、多中心研究进一步观察。
| [1] |
Harms J, Rolinger H. Die operative behandlung der spondylolisthese durch dorsale aufrichtung und ventrale verblockung[A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion(author′s transl)][J]. Z Orthop Ihre Grenzgeb, 1982, 120(3): 343-347. |
| [2] |
Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion[J]. Spine(Phila Pa 1976), 2003, 28(15 Suppl): S26-S35. |
| [3] |
Tan JH, Liu G. Is MIS-TLIF superior to open TLIF in obese patients? A systematic review and meta-analysis[J]. Eur Spine J, 2018, 28(8): 1881-1883. |
| [4] |
吴建新. 20年腰椎间盘突出症病例回顾分析及中长期随访研究[D]. 上海: 第二军医大学, 2008.
|
| [5] |
Huskisson EC. Measurement of pain[J]. Lancet, 1974, 2(7889): 1127-1131. |
| [6] |
Fairbank JC, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire[J]. Physiotherapy, 1980, 66(8): 271-273. |
| [7] |
Bridwell KH, Lenke LG, McEnery KW, et al. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects?[J]. Spine(Phila Pa 1976), 1995, 20(12): 1410-1418. DOI:10.1097/00007632-199506020-00014 |
| [8] |
Rao G, Brodke DS, Rondina M, et al. Inter- and intraobserver reliability of computed tomography in assessment of thoracic pedicle screw placement[J]. Spine(Phila Pa 1976), 2003, 28(22): 2527-2530. DOI:10.1097/01.BRS.0000092341.56793.F1 |
| [9] |
de Kunder SL, van Kuijk SMJ, Rijkers K, et al. Transforaminal lumbar interbody fusion(TLIF) versus posterior lumbar interbody fusion(PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis[J]. Spine J, 2017, 17(11): 1712-1721. DOI:10.1016/j.spinee.2017.06.018 |
| [10] |
肖波, 毛克亚, 王岩, 等. 微创经椎间孔腰椎椎体间融合术与传统后路腰椎椎体间融合术并发症的比较分析[J]. 脊柱外科杂志, 2013, 11(1): 23-27. DOI:10.3969/j.issn.1672-2957.2013.01.005 |
| [11] |
Chen K, Chen H, Zhang K, et al. O-arm navigation combined with microscope-assisted MIS-TLIF in the treatment of lumbar degenerative disease[J]. Clin Spine Surg, 2019, 32(5): E235-E240. DOI:10.1097/BSD.0000000000000804 |
| [12] |
Ge DH, Stekas ND, Varlotta CG, et al. Comparative analysis of two transforaminal lumbar interbody fusion techniques: open TLIF versus Wiltse MIS TLIF[J]. Spine(Phila Pa 1976), 2019, 44(9): E555-E560. DOI:10.1097/BRS.0000000000002903 |
| [13] |
Parrish JM, Jenkins NW, Brundage TS, et al. Outpatient minimally invasive lumbar fusion using multimodal analgesic management in the ambulatory surgery setting[J]. Int J Spine Surg, 2020, 14(6): 970-981. DOI:10.14444/7146 |
| [14] |
Zhang H, Zhou C, Wang C, et al. Percutaneous endoscopic transforaminal lumbar interbody fusion: technique note and comparison of early outcomes with minimally invasive transforaminal lumbar interbody fusion for lumbar spondylolisthesis[J]. Int J Gen Med, 2021, 14: 549-558. DOI:10.2147/IJGM.S298591 |
| [15] |
Qin R, Wu T, Liu H, et al. Minimally invasive versus traditional open transforaminal lumbar interbody fusion for the treatment of low-grade degenerative spondylolisthesis: a retrospective study[J]. Sci Rep, 2020, 10(1): 21851. DOI:10.1038/s41598-020-78984-x |
| [16] |
Kang YN, Ho YW, Chu W, et al. Effects and safety of lumbar fusion techniques in lumbar spondylolisthesis: a network meta-analysis of randomized controlled trials[J]. Global Spine J, 2021. DOI:10.1177/2192568221997804 |
| [17] |
Tsai CY, Su YF, Kuo KL, et al. Minimally invasive transforaminal lumbar interbody fusion for 2-level degenerative lumbar disease in patients with osteoporosis: long-term clinical and radiographic outcomes[J]. Oper Neurosurg(Hagerstown), 2021, 20(6): 535-540. DOI:10.1093/ons/opab009 |
| [18] |
Le H, Anderson R, Phan E, et al. Clinical and radiographic comparison between open versus minimally invasive transforaminal lumbar interbody fusion with bilateral facetectomies[J]. Global Spine J, 2020, 11(6): 903-910. |
| [19] |
Xie L, Wu WJ, Liang Y. Comparison between minimally invasive transforaminal lumbar interbody fusion and conventional open transforaminal lumbar interbody fusion: an updated meta-analysis[J]. Chin Med J(Engl), 2016, 129(16): 1969-1986. |
| [20] |
Lv Y, Chen J, Chen J, et al. Three-year postoperative outcomes between MIS and conventional TLIF in 1-segment lumbar disc herniation[J]. Minim Invasive Ther Allied Technol, 2017, 26(3): 168-176. DOI:10.1080/13645706.2016.1273837 |
| [21] |
Leonova ON, Cherepanov EA, Krutko AV. MIS-TLIF versus O-TLIF for single-level degenerative stenosis: study protocol for randomised controlled trial[J]. BMJ Open, 2021, 11(3): e041134. DOI:10.1136/bmjopen-2020-041134 |
| [22] |
Arif S, Brady Z, Enchev Y, et al. Minimising radiation exposure to the surgeon in minimally invasive spine surgeries: a systematic review of 15 studies[J]. Orthop Traumatol Surg Res, 2020, 107(7): 102795. |
| [23] |
Lee KH, Yeo W, Soeharno H, et al. Learning curve of a complex surgical technique: minimally invasive transforaminal lumbar interbody fusion(MIS TLIF)[J]. J Spinal Disord Tech, 2014, 27(7): E234-E240. DOI:10.1097/BSD.0000000000000089 |
| [24] |
Kang MS, Park JY, Kim KH, et al. Minimally invasive transforaminal lumbar interbody fusion with unilateral pedicle screw fixation: comparison between primary and revision surgery[J]. Biomed Res Int, 2014, 919248. |
| [25] |
Goertz L, Stavrinou P, Hamisch C, et al. Impact of obesity on complication rates, clinical outcomes, and quality of life after minimally invasive transforaminal lumbar interbody fusion[J]. J Neurol Surg A Cent Eur Neurosurg, 2021, 82(2): 147-153. DOI:10.1055/s-0040-1718758 |
| [26] |
Dusad T, Kundnani V, Dutta S, et al. Comparative prospective study reporting intraoperative parameters, pedicle screw perforation, and radiation exposure in navigation-guided versus non-navigated fluoroscopy-assisted minimal invasive transforaminal lumbar interbody fusion[J]. Asian Spine J, 2018, 12(2): 309-316. DOI:10.4184/asj.2018.12.2.309 |
| [27] |
Bai JY, Zhang W, An JL, et al. True anteroposterior view pedicle screw insertion technique[J]. Ther Clin Risk Manag, 2016, 12: 1039-1047. DOI:10.2147/TCRM.S99362 |
| [28] |
Venier A, Croci D, Robert T, et al. Use of intraoperative computed tomography improves outcome of minimally invasive transforaminal lumbar interbody fusion: a single-center retrospective cohort study[J]. World Neurosurg, 2021, 148: e572-e580. DOI:10.1016/j.wneu.2021.01.041 |
| [29] |
Zhao Y, Yuan S, Tian Y, et al. Risk factors related to superior facet joint violation during lumbar percutaneous pedicle screw placement in minimally invasive transforaminal lumbar interbody fusion(MIS-TLIF)[J]. World Neurosurg, 2020, 139: e716-e723. DOI:10.1016/j.wneu.2020.04.118 |
 2022, Vol.20
2022, Vol.20  Issue(4): 217-222
Issue(4): 217-222


