2. 南通大学附属南京江北医院骨科, 南京 210044;
3. 南京医科大学附属明基医院骨科, 南京 210019
2. Department of Orthopaedics, Nanjing Jiangbei Hospital, Nantong University, Nanjing 210044, Jiangsu, China;
3. Department of Orthopaedics, BenQ Hospital, Nanjing Medical University, Nanjing 210019, Jiangsu, China
随着全球老龄化进程的加快,老年骨质疏松性椎体压缩性骨折(OVCF)的发生率逐年增加[1-2],若不及时进行临床干预,极易演化为Kummell病,导致顽固性腰痛、脊柱后凸畸形等[3]。对于急性症状性OVCF,德国骨科及创伤协会(DGOU)提出骨质疏松骨折(DGOU-OF)分型及OF评分系统[4-5],建议OF 1~2型且评分 > 6分的患者行微创手术治疗,早期恢复生活自理能力。目前,以经皮椎体后凸成形术(PKP)及经皮椎体成形术(PVP)为代表的椎体强化术广泛用于OVCF的治疗[6],其可有效缓解疼痛、强化椎体,患者术后早期即可进行功能锻炼,避免发生卧床相关并发症[7]。然而,有研究[8-9]证实,OVCF手术时机的选择是影响疗效的关键因素之一。何时行椎体强化术不仅与手术的难易程度、风险大小密切相关,也影响术中骨水泥的分布、伤椎生物力学稳定性、术后远期椎体高度丢失及邻近椎体骨折的发生情况。骨折时间越长,伤椎血肿机化越容易引起骨折畸形愈合,导致伤椎高度复位不佳;同时,骨折间隙变窄而致骨水泥注入量减少、骨水泥弥散欠佳,降低伤椎生物力学稳定。因此,手术时机的选择是影响OVCF远期疗效的关键因素之一。本研究通过对比急性期和非急性期采用椎体强化术治疗的老年单节段OVCF患者围手术期及末次随访资料,探讨OVCF治疗的手术时机选择,为临床提供手术时机选择参考。
1 资料与方法 1.1 一般资料纳入标准:①年龄 > 65岁;②低能量损伤(轻微暴力外伤)导致的单节段胸腰椎骨折(T11~L5);③根据外伤史、体格检查、骨密度及MRI检查明确诊断为OVCF;④DGOU-OF分型为OF 1~2型,且OF评分 > 6分;⑤骨折时间 < 3个月。排除标准:①伴脊髓神经损伤;②椎体高度压缩比 > 40%;③严重凝血功能障碍;④伴严重内科疾病无法手术;⑤后柱损伤、椎弓根断裂移位;⑥Kummell病。
根据上述标准,纳入2018年12月—2020年6月南京医科大学第四附属医院采用椎体强化术治疗的老年单节段OVCF患者148例,按照骨折至手术时间分为急性期组(≤14 d,76例)和非急性期组(> 14 d,72例)。急性期组11例伴腰背肌筋膜炎、6例伴压疮、13例伴下肢静脉血栓(其中1例为深静脉血栓);非急性期组22例伴腰背肌筋膜炎、15例伴压疮、24例伴下肢静脉血栓(其中5例为深静脉血栓)。2组术前一般资料差异无统计学意义(P > 0.05,表 1)。
|
|
表 1 2组患者术前资料 Tab. 1 Preoperative data of 2 groups |
根据术前X线片上伤椎压缩程度,高度丢失≥25%采用PKP治疗,< 25%采用PVP治疗。
PVP治疗:常规C形臂X线机透视定位并标记伤椎两侧椎弓根。消毒、铺巾,采用局部麻醉,在距椎弓根体表标志约1 cm处做一5 mm的切口,椎弓根钻子外展合适角度平行伤椎终板插入双侧椎弓根,透视确认正侧位均在椎弓根投影内,角度、位置良好。插入导针至椎体前缘并拧入扩孔套筒,在透视下将套筒插至椎体后缘前约3 mm建立通道。然后将絮状明胶海绵塞入套筒内,并调整靠近椎体破损区域。调制骨水泥至拉丝状后向椎体内注入。手术过程中注意观察有无骨水泥渗漏,若发生渗漏则立刻停止操作并了解患者有无不适。
PKP治疗:建立穿刺通道方法同PVP。将球囊抽真空,压力泵注入造影剂后推至椎体塌陷的位置,调整压力泵进行球囊扩张,逐步复位骨折椎体高度。之后操作同PVP。
术后1 d患者可佩戴腰部支具下床活动;术后进行规范抗骨质疏松治疗,包括基础药物治疗(钙剂+维生素D)和抗骨质疏松药物治疗(每年静滴一次5 mg唑来膦酸);术后3 d复查X线片及CT,观察骨水泥分布情况及有无渗漏。
1.3 评价指标术后定期复查X线片及CT。侧位X线片示骨水泥弥散至椎体前柱,正位X线片示骨水泥弥散至椎体中央部分且分布到上下终板为骨水泥弥散良好。在侧位X线片上测量椎体高度,包括椎体前缘高度(AVH)和椎体中部高度(CVH)。椎体高度百分比(%)=(2×伤椎高度)/(上位椎体高度+下位椎体高度)×100%,术后椎体高度百分比大于术前且 > 90%定义为椎体复位良好;术后椎体高度丢失≥15%或椎体局部后凸角度增加≥10°定义为椎体高度丢失[10]。在X线片及CT上观察骨水泥渗漏情况。采用VAS评分[11]评估疼痛程度,VAS评分≥4分[12]定义为残余痛。采用ODI[13]评估功能改善情况。
1.4 统计学处理采用SPSS 26.00软件对数据进行统计分析。符合正态分布的计量资料以x±s表示,组间比较采用独立样本t检验;计数资料以频数表示,组间比较采用χ2检验;以P < 0.05为差异有统计学意义。
2 结果 2.1 围手术期所有手术顺利完成,未发生脊髓神经损伤等并发症。非急性期组5例深静脉血栓及急性期组1例深静脉血栓患者均行下肢滤器置入术后再行手术治疗。2组在手术方式、手术时间、VAS评分、ODI、骨水泥渗漏方面,差异均无统计学意义(P > 0.05,表 2);在骨水泥注入量、骨水泥弥散情况、AVH百分比、CVH百分比及复位效果方面,急性期组优于非急性期组,差异均有统计学意义(P < 0.05,表 2)。
|
|
表 2 2组围手术期临床资料 Tab. 2 Perioperative clinical data of 2 groups |
所有患者随访12~24(15.42±3.84)个月。末次随访时,2组邻近椎体骨折、骨密度T值情况,差异无统计学意义(P > 0.05,表 3);在残余痛、椎体高度丢失、VAS评分、Cobb角、AVH百分比及CVH百分比方面,急性期组优于非急性期组,差异均有统计学意义(P < 0.05,表 3)。2组典型病例影像学资料见图 1、2。
|
|
表 3 2组患者末次随访资料 Tab. 3 Final follow-up data of 2 groups |
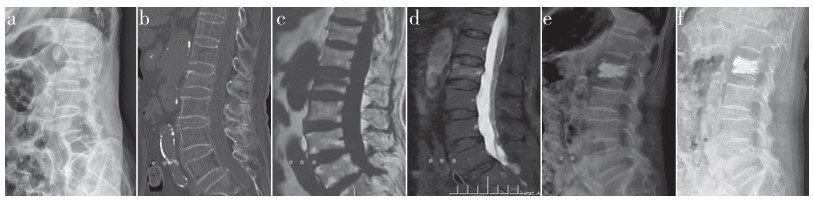
|
图 1 急性期组典型病例影像学资料 Fig. 1 Imaging data of a typical case of acute group 女,77岁,L2 OVCF,骨折至手术时间为9 d a、b:术前X线片和CT示椎体高度丢失,椎体压缩30% c、d:术前MRI T1加权像示椎体低信号,T2抑脂像示高信号 e:PKP术后X线片示骨水泥弥散良好,椎体高度恢复至96% f:末次随访X线片未见椎体塌陷 Female, 77 years old, L2 OVCF, time from fracture to operation is 9 d a, b: Preoperative roentgenograph and CT show loss of vertebral height and 30% vertebral compression c, d: Preoperative MRI T1 weight image shows hypointensity signal, T2 fat suppression image shows hyperintensity signal e: Roentgenograph after PKP shows bone cement is well dispersed, and vertebral height is restored to 96% f: Roentgenograph at final follow-up shows no vertebral collapse |
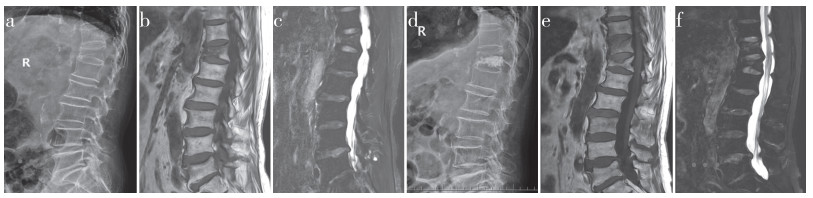
|
图 2 非急性期组典型病例影像学资料 Fig. 2 Imaging data of a typical case of non-acute group 男,70岁,T12 OVCF,骨折至手术时间为21 d a:术前X线片示T12椎体高度丢失,椎体压缩32% b、c:术前MRI T1加权像示椎体低信号,T2抑脂像示高低信号混杂 d:PKP术后X线片示骨水泥弥散欠佳(未完全填充前柱),椎体高度丢失23% e、f:术后14个月MRI示T12椎体高度丢失、T11压缩骨折及轻微后凸畸形 Male, 70 years old, T12 OVCF, time from fracture to operation is 21 d a: Preoperative roentgenograph shows loss of vertebral height and 32% vertebral height compression b, c: Preoperative MRI T1 weight image shows hypointensity signal, T2 fat suppression image shows mixed hyperintensity and hypointensity signals d: Roentgenograph after PKP shows poor dispersion of bone cement(incomplete filling of anterior column) and 23% of loss of vertebral height e, f: MRIs at postoperative 14 months show T12 vertebral height loss, T11 compression fracture with slight kyphosis |
OVCF是最常见的脆性骨折之一[14],也是导致老年致死、致残及失能的重要原因[15]。近年来,椎体强化术广泛用于OVCF的治疗,其微创、消除疼痛及快速恢复生活的能力得到临床医师的青睐。但手术时机的把握对疗效有重要影响,延误治疗可导致椎体塌陷、脊柱后凸畸形等[16]。
OVCF的治疗目的是缓解疼痛、消除失能状态、强化椎体、预防邻近椎体骨折[17]。OVCF患者往往处于失能状态,非手术治疗(制动及卧床休息、抗炎止痛)需要患者长期卧床,会引起患者胃肠蠕动减慢,导致营养进行性下降,影响骨吸收、骨形成,加速骨质流失和骨密度降低,进一步加重骨质疏松及腰背肌萎缩[18]。本研究也发现非急性期组腰背肌筋膜炎及下肢静脉血栓的发生率明显高于急性期。因此,对有手术指征的老年OVCF患者应早期行手术治疗,快速恢复生活能力,减少卧床相关并发症的发生。
骨水泥渗漏是椎体强化术最常见的并发症之一,有报道骨水泥渗漏率为55.9%[19]。骨水泥渗漏会导致伤椎生物力学性能改变,不仅使相邻椎体的最大应力增加,严重时还可造成脊髓、神经损伤甚至瘫痪等灾难性后果。有研究[20]证实,2周内的OVCF表现为血肿和局部炎性反应,2周~2个月时内生软骨骨化和骨形成,2个月以上骨组织重建。因此,理论上急性期手术骨水泥容易从椎体破损处或滋养血管渗漏,增大骨水泥渗漏的风险;而非急性期由于血肿机化,椎体破损处已有一定的屏障作用,降低了骨水泥的渗漏风险。但本研究结果显示,2组骨水泥渗漏情况无明显差异,急性期手术并不显著增加骨水泥渗漏率,分析原因为本研究术中应用明胶海绵填塞,形成机械屏障并增加了骨水泥的流动阻力[21-22]。
骨水泥弥散良好是提高手术疗效的必要条件之一[23]。骨水泥弥散不佳会影响脊柱的力学传递,同时降低伤椎生物力学稳定性及椎体力学强度,影响远期临床效果[24]。本研究结果证实,急性期组骨水泥弥散情况较非急性期组好,同时急性期组椎体高度恢复更好,远期随访椎体高度丢失较少。非急性期组患者由于骨折时间较长,伤椎会随脊柱伸屈运动而产生微运动,部分患者出现骨折愈合不良、骨硬化甚至假关节形成,并且伴有炎性反应及腰背肌筋膜炎,导致痛觉过敏。因此,有研究[25]报道,手术时间过晚存在疼痛缓解欠佳等缺点。本研究发现,随着骨折时间的延长,骨水泥注入量逐渐减少,骨水泥弥散不良的比例增加;分析原因是骨折后血肿机化及骨折畸形愈合阻碍了骨水泥与松质骨的接触,限制了骨水泥的弥散,降低了骨水泥与骨组织的结合能力。因此,有研究[26]报道,骨水泥弥散度随骨折时间的延长而降低。
椎体强化术治疗OVCF,术后远期随访发生伤椎高度丢失及邻近椎体骨折是一直困扰骨科医师的难题,如何有效预防椎体高度丢失及椎体再发骨折是维持远期疗效的关键。OVCF急性期间伤椎内为血肿或肉芽组织,压缩的椎体更容易被球囊撑开复位,且复位后仍有骨小梁框架支撑,弥散良好的骨水泥与骨小梁紧密结合,可维持脊柱生物力学稳定性,降低远期椎体高度丢失的风险[27]。而OVCF非急性期间骨折端已纤维愈合,复位椎体压力较大;同时,部分患者骨折后仍负重活动,导致伤椎进一步压缩,术中椎体高度更难恢复甚至无法复位。本研究结果显示,急性期组骨水泥注入量明显高于非急性期组,椎体高度恢复明显优于非急性期组,末次随访时非急性期组7例发生邻近椎体骨折、16例发生椎体高度丢失,发生率均高于急性期组。
综上所述,急性期OVCF采用椎体强化术治疗,可有效缓解疼痛、快速恢复患者生活能力,避免卧床相关并发症发生,术中骨水泥弥散情况良好,可有效复位伤椎高度;同时,远期随访期间椎体高度丢失、邻近椎体骨折及残余痛的发生率较低。但本研究纳入样本量少、随访时间短,结果可能存在偏倚,今后有待增加样本量及随访时间,进一步完善研究结论。
| [1] |
张保良, 陈允震. 骨质疏松性椎体压缩骨折住院患者的人口学特征及临床特征分析[J]. 中华骨科杂志, 2019, 39(24): 1523-1535. DOI:10.3760/cma.j.issn.0253-2352.2019.24.005 |
| [2] |
Sanli I, van Kuijk SMJ, de Bie RA, et al. Percutaneous cement augmentation in the treatment of osteoporotic vertebral fractures(OVFs) in the elderly: a systematic review[J]. Eur Spine J, 2020, 29(7): 1553-1572. DOI:10.1007/s00586-020-06391-x |
| [3] |
Lim J, Choi SW, Youm JY, et al. Posttraumatic delayed vertebral collapse: kummell's disease[J]. J Korean Neurosurg Soc, 2018, 61(1): 1-9. DOI:10.3340/jkns.2017.0505.010 |
| [4] |
Schnake KJ, Blattert TR, Hahn P, et al. Classification of osteoporotic thoracolumbar spine fractures: recommendations of the Spine Section of the German Society for Orthopaedics and Trauma(DGOU)[J]. Global Spine J, 2018, 8(2 Suppl): 46S-49S. |
| [5] |
Blattert TR, Schnake KJ, Gonschorek O, et al. Nonsurgical and surgical management of osteoporotic vertebral body fractures: recommendations of the Spine Section of the German Society for Orthopaedics and Trauma(DGOU)[J]. Global Spine J, 2018, 8(2 Suppl): 50S-55S. |
| [6] |
中华医学会骨科学分会骨质疏松学组. 骨质疏松性骨折诊疗指南[J]. 中华骨科杂志, 2017, 37(1): 1-10. DOI:10.3760/cma.j.issn.0253-2352.2017.01.001 |
| [7] |
中国医师协会骨科学分会脊柱创伤专业委员会. 急性症状性骨质疏松性胸腰椎压缩骨折椎体强化术临床指南[J]. 中华创伤杂志, 2019, 35(6): 481-489. DOI:10.3760/cma.j.issn.1001-8050.2019.06.001 |
| [8] |
Van Meirhaeghe J, Bastian L, Boonen S, et al. A randomized trial of balloon kyphoplasty and nonsurgical management for treating acute vertebral compression fractures: vertebral body kyphosis correction and surgical parameters[J]. Spine (Phila Pa 1976), 2013, 38(12): 971-983. DOI:10.1097/BRS.0b013e31828e8e22 |
| [9] |
Yang EZ, Xu JG, Huang GZ, et al. Percutaneous vertebroplasty versus conservative treatment in aged patients with acute osteoporotic vertebral compression fractures: a prospective randomized controlled clinical study[J]. Spine(Phila Pa 1976), 2016, 41(8): 653-660. DOI:10.1097/BRS.0000000000001298 |
| [10] |
Yu W, Liang D, Yao Z, et al. Risk factors for recollapse of the augmemed vertebrae after percutaneous vertebmplasty for osteoporotic vertebral fractures with intravertebral vacuum cleft[J]. Medicine(Baltimore), 2017, 96(2): e5675. |
| [11] |
Huskisson EC. Measurement of pain[J]. Lancet, 1974, 2(7889): 1127-1131. |
| [12] |
Li Y, Yue J, Huang M, et al. Risk factors for postoperative residual back pain after percutaneous kyphoplasty for osteoporotic vertebral compression fractures[J]. Eur Spine J, 2020, 29(10): 2568-2575. DOI:10.1007/s00586-020-06493-6 |
| [13] |
Fairbank JC, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire[J]. Physiotherapy, 1980, 66(8): 271-273. |
| [14] |
中华医学会骨质疏松和骨矿盐疾病分会. 骨质疏松性椎体压缩性骨折诊疗与管理专家共识[J]. 中华骨质疏松和骨矿盐疾病杂志, 2018, 11(5): 425-437. DOI:10.3969/j.issn.1674-2591.2018.05.001 |
| [15] |
Sebaaly A, Nabhane L, Issa EL, et al. Vertebral augmentation: state of the art[J]. Asian Spine J, 2016, 10(2): 370-376. DOI:10.4184/asj.2016.10.2.370 |
| [16] |
Park SJ, Kim HS, Lee SK, et al. Bone cement-augmented percutaneous short segment fixation: an effective treatment for Kummell's disease?[J]. J Korean Neurosurg Soc, 2015, 58(1): 54-59. DOI:10.3340/jkns.2015.58.1.54 |
| [17] |
Cheng X, Long HQ, Xu JH, et al. Comparison of unilateral versus bilateral percutaneous kyphoplasty for the treatment of patients with osteoporosis vertebral compression fracture(OVCF): a systematic review and meta-analysis[J]. Eur Spine J, 2016, 25(11): 3439-3449. DOI:10.1007/s00586-016-4395-6 |
| [18] |
Noriega DC, Ramajo RH, Lite IS, et al. Safety and clinical performance of kyphoplasty and SpineJack(®)procedures in the treatment of osteoporotic vertebral compression fractures: a pilot, monocentric, investigator-initiated study[J]. Osteoporos Int, 2016, 27(6): 2047-2055. DOI:10.1007/s00198-016-3494-x |
| [19] |
王惠东, 姚方超, 傅智轶, 等. 经皮椎体成形术治疗老年骨质疏松性胸腰椎压缩性骨折术中骨水泥渗漏的相关因素[J]. 脊柱外科杂志, 2019, 17(3): 192-197. DOI:10.3969/j.issn.1672-2957.2019.03.009 |
| [20] |
Diamond TH, Clark WA, Kumar SV. Histomorphometric analysis of fracture healing cascade in acute osteoporotic vertebral body fractures[J]. Bone, 2007, 40(3): 775-780. DOI:10.1016/j.bone.2006.10.009 |
| [21] |
Liu H, Wang S, Liu T, et al. Incremental temperature cement delivery technique may prevent cement leakage in metastatic vertebral lesions[J]. J Orthop Surg(Hong Kong), 2017, 25(3): 2309499017718931. |
| [22] |
Park JH, Kim HS, Kim SW. Cement leakage into adjacent vertebral body following percutaneous vertebroplasty[J]. Korean J Spine, 2016, 13(2): 74-76. DOI:10.14245/kjs.2016.13.2.74 |
| [23] |
张大鹏, 毛克亚, 强晓军, 等. 弯角经皮椎体成形术治疗骨质疏松性椎体压缩性骨折术后的骨水泥分布[J]. 脊柱外科杂志, 2022, 20(2): 121-124. |
| [24] |
Yang S, Liu Y, Yang H, et al. Risk factors and correlation of secondary adjacent vertebral compression fracture in percutaneous kyphoplasty[J]. Int J Surg, 2016, 36(Pt A): 138-142. |
| [25] |
Svensson HK, Olsson LE, Hansson T, et al. The effects of person-centered or other supportive interventions in older women with osteoporotic vertebral compression fractures—a systematic review of the literature[J]. Osteoporosis Int, 2017, 28(9): 2521-2540. DOI:10.1007/s00198-017-4099-8 |
| [26] |
刘长枫, 宋文慧, 刘昌文, 等. 经皮椎体成形术骨水泥分布评价及影响因素分析[J]. 中国脊柱脊髓杂志, 2019, 29(11): 1001-1008. |
| [27] |
Chen LH, Hsieh MK, Liao JC, et al. Repeated percutaneous vertebroplasty for refracture of cemented vertebrae[J]. Arch Orthop Trauma Surg, 2011, 131(7): 927-933. DOI:10.1007/s00402-010-1236-7 |
 2023, Vol.21
2023, Vol.21  Issue(1): 7-12
Issue(1): 7-12


